LiNi1/3Mn1/3Co1/3O2 nanoparticles produced by flame spray pyrolysis with crystallinity characteristics similar to commercial NMC particles
- PMID: 40778098
- PMCID: PMC12329498
- DOI: 10.1039/d5ra02976g
LiNi1/3Mn1/3Co1/3O2 nanoparticles produced by flame spray pyrolysis with crystallinity characteristics similar to commercial NMC particles
Abstract
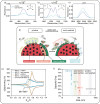
To achieve higher energy densities in lithium-ion batteries, improvements in the battery cathode performance are crucial. As cathode materials, nickel-rich layered transition metal oxides play an important role in the market. However, they suffer from surface degradation which contributes to the capacity fade. Using nanoparticles, which offer a large surface to volume ratio, these surface degradation reactions can be better understood. But to do so, nanoparticles with properties similar to those of primary particles in commercial NMC are necessary. In this work, we present the synthesis of sub-100 nm of LiNi1/3Mn1/3Co1/3O2 (NMC111) nanoparticles through a flame spray pyrolysis and post-calcination. We study the phase purity and electrochemical performance of the NMC111 nanoparticles as a function of the calcination temperature and demonstrate that optimizing the calcination temperature enables us to achieve a pure layered phase and electrochemical performance on par with commercial NMC111 particles. Mild acid treatment can be used to remove surface impurities that develop with air exposure and improve the long-term stability.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Camargos P. H. dos Santos P. H. J. dos Santos I. R. Ribeiro G. S. Caetano R. E. Perspectives on Li-Ion Battery Categories for Electric Vehicle Applications: A Review of State of the Art. Int. J. Energy Res. 2022;25:19258–19268.
-
- Kim J. M. Zhang X. Zhang J. G. Manthiram A. Meng Y. S. Xu W. A Review on the Stability and Surface Modification of Layered Transition-Metal Oxide Cathodes. Mater. Today. 2021;46:155–182.
-
- Xu J. Deshpande R. D. Pan J. Cheng Y.-T. Battaglia V. S. Electrode Side Reactions, Capacity Loss and Mechanical Degradation in Lithium-Ion Batteries. J. Electrochem. Soc. 2015;162(10):A2026–A2035.
-
- Morin H. R. Graczyk D. G. Tsai Y. Lopykinski S. Iddir H. Garcia J. C. Dietz Rago N. Trask S. Flores L. R. Son S. B. Zhang Z. Johnson N. M. Bloom I. Transition-Metal Dissolution from NMC-Family Oxides: A Case Study. ACS Appl. Energy Mater. 2020;3(3):2565–2575.
-
- Duan Y. Yang L. Zhang M. J. Chen Z. Bai J. Amine K. Pan F. Wang F. Insights into Li/Ni Ordering and Surface Reconstruction during Synthesis of Ni-Rich Layered Oxides. J. Mater. Chem. A. 2019;7(2):513–519.
LinkOut - more resources
Full Text Sources
Research Materials

