Design, synthesis, and antimicrobial evaluation of novel 1,2,4-trizaole thioether derivatives with a 1,3,4-thiadiazole skeleton
- PMID: 40778099
- PMCID: PMC12329654
- DOI: 10.1039/d5ra04574f
Design, synthesis, and antimicrobial evaluation of novel 1,2,4-trizaole thioether derivatives with a 1,3,4-thiadiazole skeleton
Abstract
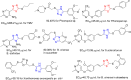
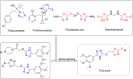
The aim of this study was to design and synthesize 17 novel 1,2,4-triazole thioether derivatives containing 1,3,4-thiadiazole thioether. Bioactivity assays indicated that several target compounds exhibited moderate to good antifungal and antibacterial activities. Notably, compound 9d demonstrated significantly stronger antifungal activity against Trichoderma sp. in Morchella esculenta (TSM) and Mucor sp. in Dictyophora rubrovalvata (MSD), with EC50 values of 9.25 and 12.95 μg mL-1, respectively, compared to the commercial fungicide pyrimethanil, which had EC50 values of 35.29 and 15.51 μg mL-1. Furthermore, compound 9d found to have strong antibacterial activity against Pseudomonas syringae pv. actinidiae (PSA) in vitro, with an EC50 of 9.34 μg mL-1, markedly superior to that of thiodiazole copper (81.74 μg mL-1). Additionally, the in vivo anti-PSA assessment revealed therapeutic and protective activities at 200 μg mL-1 of 59.39% and 79.33%, respectively, outperforming thiodiazole copper (38.91% and 67.64%, respectively). According to molecular docking simulations the interaction between compound 9d and FtsZ was mainly due to hydrogen bond formations with GLU-296, ARG-301, LYS-188, ASP-29 and GLY-30. This report suggests that these novel 1,2,4-triazole thioether derivatives featuring a 1,3,4-thiadiazole skeleton may effectively mitigate fungal and bacterial threats to plants.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Design, Synthesis, and Antifungal Activity Evaluation of Novel Hydrazide-Containing L-Perillaldehyde Derivatives as Potential Fungicides.J Agric Food Chem. 2025 Aug 25. doi: 10.1021/acs.jafc.5c02794. Online ahead of print. J Agric Food Chem. 2025. PMID: 40853852
-
Novel 5,6-dihydrobenzo[h]quinazoline derivatives as succinate dehydrogenase inhibitors: design, synthesis, antifungal activity and mechanism of action.Pest Manag Sci. 2025 Jul 4. doi: 10.1002/ps.70025. Online ahead of print. Pest Manag Sci. 2025. PMID: 40613336
-
Novel 2,5-dihydro-3H-[1,2,4]triazino[5,6-b]indole derivatives decorated with disulfide moiety are effective for treating bacterial infections by inducing reactive oxygen species.Pest Manag Sci. 2025 Sep;81(9):5413-5427. doi: 10.1002/ps.8895. Epub 2025 May 14. Pest Manag Sci. 2025. PMID: 40364659
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
References
-
- Khan B. A., Nadeem M. A., Nawaz H., Amin M. M., Abbasi G. H., Nadeem M., Ali M., Ameen M., Javaid M. M., Maqbool R., Ikram M. and Ayub M. A., Pesticides: Impacts on Agriculture Productivity, Environment, and Management Strategies, Springer, Cham, p. 2023
-
- Li B. Yin X. Cen B. Duan W. Lin G. Wang X. Zou R. Nat. Prod. Res. 2022;38:359. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

