This is a preprint.
The PREVENT-AD cohort: accelerating Alzheimer's disease research and treatment in Canada and beyond
- PMID: 40778177
- PMCID: PMC12330406
- DOI: 10.1101/2025.07.22.25331791
The PREVENT-AD cohort: accelerating Alzheimer's disease research and treatment in Canada and beyond
Update in
-
The PREVENT-AD cohort: Accelerating Alzheimer's disease research and treatment in Canada and beyond.Alzheimers Dement. 2025 Oct;21(10):e70653. doi: 10.1002/alz.70653. Alzheimers Dement. 2025. PMID: 41020412 Free PMC article.
Abstract
The PREVENT-AD is an investigator-driven study that was created in 2011 and enrolled cognitively normal older adults with a family history of sporadic AD. Participants are deeply phenotyped and have now been followed annually for more than 12 years [median follow-up 8.0 years,SD 3.1]. Multimodal MRI, genetic, neurosensory, clinical, cerebrospinal fluid and cognitive data collected until 2017 on 348 participants who agreed to open sharing with the neuroscience community were already available. We now share a new release including 6 years of additional follow-up cognitive data, and additional MRI follow-ups, clinical progression, new longitudinal behavioral and lifestyle measures (questionnaires, actigraphy), longitudinal AD plasma biomarkers, amyloid-beta and tau PET, magnetoencephalography, as well as neuroimaging analytic measures from all MRI modalities. We describe the PREVENT-AD study, the data shared with the global research community as well as the model we created to sustain longitudinal follow-ups while also allowing new innovative data collection.
Keywords: Biomarkers; clinical progression; cognition; data repository; neuroimaging; preclinical.
Conflict of interest statement
CONFLICTS OF INTEREST KB has served as a consultant and at advisory boards for Abbvie, AC Immune, ALZPath, AriBio, Beckman-Coulter, BioArctic, Biogen, Eisai, Lilly, Moleac Pte. Ltd, Neurimmune, Novartis, Ono Pharma, Prothena, Quanterix, Roche Diagnostics, Sanofi and Siemens Healthineers; has served at data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai and Roche Diagnostics; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. The other authors report no competing interests
Figures
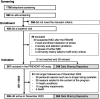


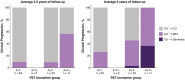
References
-
- Breitner JCS, Poirier J, Etienne PE, Leoutsako JM, Group. ftP-AR. Rational and structure for a new center for studies on prevention of Alzheimer’s disease (STOP-AD). The Journal of Prevention of Alzheimer’s Disease. 2016;3(4):236–242. - PubMed
-
- Duff K, Humphreys Clark JD, O’Bryant SE, Mold JW, Schiffer RB, Sutker PB. Utility of the RBANS in detecting cognitive impairment associated with Alzheimer’s disease: sensitivity, specificity, and positive and negative predictive powers. Arch Clin Neuropsychol. Sep 2008;23(5):603–12. doi: 10.1016/j.acn.2008.06.004 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
