Decoding the Interplay of Hydrogen Bonding, Dispersion, and Steric Interactions in Conformational Isomerism Among Functionalized Pillar[n]arenes
- PMID: 40778213
- PMCID: PMC12330302
- DOI: 10.1021/acs.jpcc.4c05974
Decoding the Interplay of Hydrogen Bonding, Dispersion, and Steric Interactions in Conformational Isomerism Among Functionalized Pillar[n]arenes
Abstract
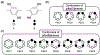
Pillar[n]arenes have garnered popularity due to their unique pillar-shaped structure, which results in hydrophobic cavities. These cavities facilitate the formation of inclusion complexes with guest molecules through non-covalent interactions such as π - π stacking, hydrogen bonding, and van der Waals interactions. Such host-guest interactions enable diverse functionalities in pillar[n]arenes, including molecule recognition, self-assembly, and encapsulation. Nevertheless, it is important to note that the host-guest properties of pillar[n]arenes can be influenced by conformational changes, primarily driven by the rotation of hydroquinone units about their methylene bridge axis. These structural changes can lead to variations in underlying non-covalent and steric interactions, impacting the overall stability of the host-guest system and potentially leading to selective uptake of guest molecules. Additionally, due to relative energy differences, we expect a distribution of pillar[n]arene conformations at thermal equilibrium. In this work, we employ density functional theory (DFT) to evaluate ground state electronic structures of pillar[n]arene conformations across pillar[n]arenes of various sizes and functionalizations. We have aimed to explore the impact of dispersion interactions, hydrogen bonding, and steric interactions on the overall energetics of pillar[n]arene conformations and determine the dominant conformation at 298 K using a Boltzmann-weighted distribution. The relative strengths of hydrogen bonds across various pillar[n]arene conformations have been examined using Bader's QTAIM topological analysis. Furthermore, we have also assessed the solvation of pillar[n]arenes in water using an implicit solvent model that unveils quantitative distinctions in hydrogen bonding and relative dispersion contributions among various pillar[n]arene conformations. Finally, pillar[n]arene conformations with more complex functional groups such as primary amine, alkyl bromide and carboxylic acid, have been studied to evaluate the interplay between underlying interactions such as hydrogen bonding, dispersion, and steric interactions, and their collective impact on the structure and energetics of pillar[n]arene conformations.
Keywords: adsorbents; conformers; macrocyclic materials; pillar[n]arenes.
Conflict of interest statement
The authors declare no competing interest.
Figures





Similar articles
-
Zigzag-Type Molecular Belts: Synthesis, Structure, and Properties.Acc Chem Res. 2025 Aug 19;58(16):2573-2585. doi: 10.1021/acs.accounts.5c00328. Epub 2025 Jul 2. Acc Chem Res. 2025. PMID: 40601855
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Oladeji O; Saitas M; Mustapha T; Johnson NM; Chiu WA; Rusyn I; Robinson AL; Presto AA Air Pollutant Patterns and Human Health Risk following the East Palestine, Ohio, Train Derailment. Environmental Science & Technology Letters 2023, 10 (8), 680–685. DOI: 10.1021/acs.estlett.3c00324. - DOI - PMC - PubMed
-
- Wang B; Heng L; Sui Q; Peng Z; Xiao X; Zheng M; Hu J; Fiedler H; Barceló D; Yu G Insight of chemical environmental risk and its management from the vinyl chloride accident. Frontiers of Environmental Science & Engineering 2023, 17 (4), 52. DOI: 10.1007/s11783-023-1652-x. - DOI
-
- Mor S; Ravindra K; Dahiya R; Chandra A Leachate characterization and assessment of groundwater pollution near municipal solid waste landfill site. Environmental monitoring and assessment 2006, 118, 435–456. - PubMed
-
- Masoner JR; Kolpin DW; Furlong ET; Cozzarelli IM; Gray JL; Schwab EA Contaminants of emerging concern in fresh leachate from landfills in the conterminous United States. Environmental Science: Processes & Impacts 2014, 16 (10), 2335–2354. - PubMed
-
- Rasmussen JJ; Wiberg-Larsen P; Baattrup-Pedersen A; Cedergreen N; McKnight US; Kreuger J; Jacobsen D; Kristensen EA; Friberg N The legacy of pesticide pollution: an overlooked factor in current risk assessments of freshwater systems. Water Research 2015, 84, 25–32. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
