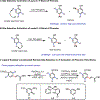
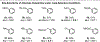Ligand-Controlled Nondirected meta- or para-C-H Olefination of Silyl-Protected Phenols
- PMID: 40778259
- PMCID: PMC12330292
- DOI: 10.1021/acscatal.4c03858
Ligand-Controlled Nondirected meta- or para-C-H Olefination of Silyl-Protected Phenols
Abstract
Recent advances in ligand design have enabled Pd(II)-catalyzed non-directed C-H functionalization using arenes as the limiting reagent, but achieving catalyst control over the site-selectivity in these transformations remains a significant challenge. Instead, selectivity is typically governed by the inherent steric and electronic properties of the arene substrates or directing effects. Consequently, it can be difficult to selectively functionalize para-position of electron-deficient arenes and meta-positions of electron-rich arenes respectively. In this report, we demonstrate that the choice of ligand in a Pd(II)-catalyzed olefination can switch selectivity between the activated para- and deactivated meta-C-H bonds of silyl-protected phenols, highly enabling site-selective functionalization of either position with broad substrate scopes. Specifically, monodentate 2-pyridone ligands enable high-yielding olefination with the conventional para-selectivity, largely governed by the intrinsic electronic bias of the substrate, whereas a dual-ligand system consisting of a bidentate pyridine-pyridone ligand and a monodentate pyridine ligand reversed the site selectivity to favor olefination of the relatively electron-deficient meta-position. Mechanistic studies indicate that the dual ligand system selectively renders para-C-H palladation reversible, but not the meta-C-H palladation, thereby favoring the meta-C-H olefination of electron-rich arenes.
Keywords: C−H activation; ligand design; non-directed; olefination; palladium; phenols.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
-
(a) For reviews on Pd-catalyzed C–H functionalization, see:
- Lyons TW; Sanford MS Palladium-Catalyzed Ligand-Directed C–H Functionalization Reactions. Chem. Rev 2010, 110, 1147–1169. - PMC - PubMed
- Chen X; Engle KM; Wang D-H; Yu J-Q Palladium(II)-Catalyzed C–H Activation/C–C Cross-Coupling Reactions: Versatility and Practicality. Angew. Chem., Int. Ed 2009, 48, 5094–5115. - PMC - PubMed
- Daugulis O; Roane J; Tran LD Bidentate, Monoanionic Auxiliary-Directed Functionalization of Carbon–Hydrogen Bonds. Acc. Chem. Res 2015, 48, 1053–1064. - PMC - PubMed
-
-
- Leow D; Li G; Mei TS; Yu J-Q Activation of Remote meta-C−H Bonds Assisted by an End-on Template. Nature 2012, 486, 518–522. - PMC - PubMed
- Chu L; Shang M; Tanaka K; Chen Q; Pissarnitski N; Streckfuss E; Yu J-Q Remote meta-C–H Activation Using a Pyridine-Based Template: Achieving Site-Selectivity via the Recognition of Distance and Geometry. ACS Cent. Sci 2015, 1, 394–399. - PMC - PubMed
- Lam NYS; Fan ZL; Wu K; Park HS; Shim SY; Strassfeld DA; Yu J-Q Empirical Guidelines for the Development of Remote Directing Templates through Quantitative and Experimental Analyses. J. Am. Chem. Soc 2022, 144, 2793–2803. - PMC - PubMed
- Xu J; Chen J; Gao F; Xie S; Xu X; Jin Z; Yu J-Q Sequential Functionalization of meta-C–H and Ipso-C–O Bonds of Phenols. J. Am. Chem. Soc 2019, 141, 1903–1907. - PMC - PubMed
-
- Zhang ZP; Tanaka K; Yu J-Q Remote Site-Selective C–H Activation Directed by a Catalytic Bifunctional Template. Nature 2017, 543, 538–542. - PMC - PubMed
- Shi H; Lu Y; Weng J; Bay KL; Chen X; Tanaka K; Verma P; Houk KN; Yu J-Q Differentiation and Functionalization of Remote C–H Bonds in Adjacent Positions. Nat. Chem 2020, 12, 399–404. - PMC - PubMed
- Kuninobu Y; Ida H; Nishi M; Kanai M A meta-Selective C−H Borylation Directed by a Secondary Interaction Between Ligand and Substrate. Nat. Chem 2015, 7, 712–717. - PubMed
- Davis HJ; Mihai MT; Phipps RJ Ion Pair-Directed Regiocontrol in Transition Metal Catalysis: A meta-Selective C–H Borylation of Aromatic Quaternary Ammonium Salts. J. Am. Chem. Soc 2016, 138, 12759–12762. - PubMed
- Yang L; Uemura N; Nakao Y meta-Selective C−H Borylation of Benzamides and Pyridines by an Iridium−Lewis Acid Bifunctional Catalyst. J. Am. Chem. Soc 2019, 141, 7972–7979. - PubMed
- Hoque ME; Bisht R; Haldar C; Chattopadhyay B Noncovalent Interactions in Ir-Catalyzed C–H Activation: L-Shaped Ligand for para-Selective Borylation of Aromatic Esters. J. Am. Chem. Soc 2017, 139, 7745–7748. - PubMed
- Zhang T; Luan Y-X; Lam NYS; Li J-F; Li Y; Ye M; Yu J-Q A Directive Ni Catalyst Overrides Conventional Site Selectivity in Pyridine C–H Alkenylation. Nat. Chem 2021, 13, 1207–1213. - PMC - PubMed
- Bisht R; Hoque ME; Chattopadhyay B Amide Effects in C−H Activation: Noncovalent Interactions with L-Shaped Ligand for meta Borylation of Aromatic Amides. Angew. Chem. Int. Ed 2018, 57, 15762–15766. - PubMed
-
- Li R; Dong G-B Structurally Modified Norbornenes: A Key Factor to Modulate Reaction Selectivity in the Palladium/Norbornene Cooperative Catalysis. J. Am. Chem. Soc 2020, 142, 17859–17875. - PMC - PubMed
- Liu LY; Qiao JX; Yeung K-S; Ewing WR Yu J-Q meta-C−H Arylation of Electron-Rich Arenes: Reversing the Conventional Site Selectivity. J. Am. Chem. Soc 2019, 141, 14870–14877. - PMC - PubMed
- Luo J; Preciado S; Larrosa I Overriding ortho−para Selectivity via a Traceless Directing Group Relay Strategy: The meta-Selective Arylation of Phenols. J. Am. Chem. Soc 2014, 136, 4109–4112. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources