Brief Report: Switching to Long-Acting CAB/RPV: Data From an Italian Monocentric Cohort
- PMID: 40778517
- PMCID: PMC11500691
- DOI: 10.1097/QAI.0000000000003501
Brief Report: Switching to Long-Acting CAB/RPV: Data From an Italian Monocentric Cohort
Abstract
Background: Cabotegravir (CAB)/rilpivirine (RPV) is the first long-acting injectable (LAI) antiretroviral therapy approved for virologically suppressed adults with HIV-1.
Setting: Italian single centre cohort.
Methods: We conducted a retrospective observational study to assess the durability, adherence to the prescribed injection schedule, and reasons for discontinuation of CAB/RPV LAI administered every 8 weeks (Q8W).
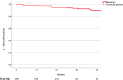
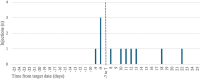
Results: One hundred thirty-eight patients were included with a median observation period of 43 weeks [interquartile range (IQR) 34-47 weeks]. Of these, 32 (23.2%) were female, and the median age was 51 years (IQR 40-58 years). Twelve patients (8.7%) discontinued CAB/RPV LAI treatment with a median time to discontinuation of 21 weeks (IQR 12-35 weeks), and 92.8% of the injections occurred within the CAB/RPV LAI schedule. The most common reason for discontinuation was injection-related pain (5/12). No confirmed virological failure occurred during the period of observation with 3 individuals who experienced virological blips.
Conclusions: Our findings showed that CAB/RPV LAI Q8W is tolerated well in clinical practice, with high adherence to the injection schedule and few discontinuations mainly related to injection site-related pain.
Keywords: HIV; adherence; cabotegravir; discontinuation; long-acting antiretroviral therapy; rilpivirine.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no funding or conflicts of interest to disclose. All authors have reviewed and approved the final version of this manuscript and affirm that this work is free from any potential conflicts of interest.
Figures
References
-
- FDA Approves Cabenuva and Vocabria for the Treatment of HIV-1 Infection. FDA. Available at: https://www.fda.gov/drugs/human-immunodeficiency-virus-hiv/fda-approves-.... Accessed January 8, 2024.
-
- Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390:1499–1510. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical