AAV-Mediated Expression of Methamphetamine Monoclonal Antibody Attenuates Methamphetamine Behaviour Sensitization in Mice
- PMID: 40778549
- PMCID: PMC12332764
- DOI: 10.1111/adb.70073
AAV-Mediated Expression of Methamphetamine Monoclonal Antibody Attenuates Methamphetamine Behaviour Sensitization in Mice
Abstract
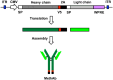
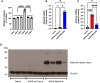
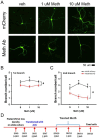
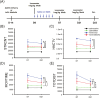
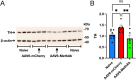
Methamphetamine (Meth) is a psychoactive and neurotoxic chemical. Selective antibodies against Meth molecules have been examined for the treatment of Meth abuse through immunization. Antibodies with high affinity for Meth can capture Meth molecules and reduce Meth response. We previously reported that intraperitoneal administration of adeno-associated virus serotype vector serotype 8 carrying Meth-specific monoclonal antibody transgene (AAV8-MethAb, 2.5 × 1010 VGC per mouse) induced long-term and stable expression of Meth-antibody in the peripheral. Mice receiving AAV8-MethAb had a lower Meth level in the blood and brain and attenuated Meth-induced locomotor activity after an acute dose of Meth. The effect of AAV-MethAb in animals receiving repeated Meth administration was still not known. In this study, we first investigated the tropism of AAV serotypes in rat primary dopaminergic (DA) neuronal culture. We found that AAV6 is an optimal gene carrier for MethAb. AAV6-MethAb or AAV6-mCherry was used in cellular and animal models of chronic Meth use. In primary DA neuronal culture, repeated Meth administration increased the dendritic branching of DA neurons, which was antagonized by AAV6-MethAb. AAV6-MethAb or AAV6-mCherry was stereotaxically administered to the nucleus accumbens (NAc) of adult CD1 mice. Two weeks after the viral injection, animals were stimulated with a daily dose of Meth for 7 days. Repeat Meth administrations led to a progressive increase in locomotor activity or behaviour sensitization. This response was significantly attenuated in mice receiving AAV6-MethAb. Using qRTPCR and Western analysis, we demonstrated that MethAb mRNA and protein were expressed in the NAc. Previous reports indicated that Meth sensitization was associated with upregulation of tyrosine hydroxylase (TH) in the NAc. Using Western blot analysis, we found that AAV6-MethAb significantly reduced TH protein levels in Meth-sensitized mice. Taken together, our data support that intracerebral administration of AAV6-MethAb reduced Meth sensitization. Our data support a novel antibody gene therapy for Meth abuse.
Keywords: adeno‐associated virus; antibody therapy; methamphetamine; sensitization.
© 2025 The Author(s). Addiction Biology published by John Wiley & Sons Ltd on behalf of Society for the Study of Addiction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Kalivas P. W. and Volkow N. D., “The Neural Basis of Addiction: A Pathology of Motivation and Choice,” American Journal of Psychiatry 162, no. 8 (2005): 1403–1413. - PubMed
-
- Hirabayasi M., Saito T., and Tadokoro S., “Differential Sensitization to Ambulation‐Increasing Effect of Methamphetamine After Repeated Administration to Mice in Activity Cages of Different Sizes,” Japanese Journal of Pharmacology 57, no. 1 (1991): 91–97. - PubMed
-
- Ujike H. and Sato M., “Clinical Features of Sensitization to Methamphetamine Observed in Patients With Methamphetamine Dependence and Psychosis,” Annals of the New York Academy of Sciences 1025 (2004): 279–287. - PubMed
-
- Chao J. and Nestler E. J., “Molecular Neurobiology of Drug Addiction,” Annual Review of Medicine 55 (2004): 113–132. - PubMed
-
- Nestler E. J., “Molecular Basis of Long‐Term Plasticity Underlying Addiction,” Nature Reviews. Neuroscience 2, no. 2 (2001): 119–128. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

