Long-term live imaging, cell identification and cell tracking in regenerating crustacean legs
- PMID: 40778836
- PMCID: PMC12334163
- DOI: 10.7554/eLife.107534
Long-term live imaging, cell identification and cell tracking in regenerating crustacean legs
Abstract
High-resolution live imaging of regeneration presents unique challenges due to the nature of the specimens (large mobile animals), the duration of the process (spanning days or weeks), and the fact that cellular resolution must be achieved without damage caused by lengthy exposures to light. Building on previous work that allowed us to image different parts of the process of leg regeneration in the crustacean Parhyale hawaiensis, we present here a method for live imaging that captures the entire process of leg regeneration, spanning up to 10 days, at cellular resolution. Our method includes (1) mounting and long-term live imaging of regenerating legs under conditions that yield high spatial and temporal resolution but minimise photodamage, (2) fixing and in situ staining of the regenerated legs that were imaged, to identify cell fates, and (3) computer-assisted cell tracking to determine the cell lineages and progenitors of identified cells. The method is optimised to limit light exposure while maximising tracking efficiency. Combined with appropriate cell-type-specific markers, this method may allow the description of cell lineages for every regenerated cell type in the limb.
Keywords: HCR; Parhyale hawaiensis; cell fate; cell lineage; developmental biology; hybridisation chain reaction; leg regeneration; live imaging.
© 2025, Çevrim, Laplace-Builhé, Sugawara et al.
Conflict of interest statement
ÇÇ, BL, MR, NL, JB, AA, MA No competing interests declared, KS KS is employed part-time by LPIXEL Inc
Figures
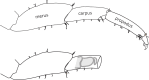









Update of
- doi: 10.1101/2024.09.11.612529
- doi: 10.7554/eLife.107534.1
Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Ventilation tubes (grommets) for otitis media with effusion (OME) in children.Cochrane Database Syst Rev. 2023 Nov 15;11(11):CD015215. doi: 10.1002/14651858.CD015215.pub2. Cochrane Database Syst Rev. 2023. PMID: 37965944 Free PMC article.
-
Antibiotics for otitis media with effusion (OME) in children.Cochrane Database Syst Rev. 2023 Oct 23;10(10):CD015254. doi: 10.1002/14651858.CD015254.pub2. Cochrane Database Syst Rev. 2023. PMID: 37870130 Free PMC article.
-
Psychological therapies for panic disorder with or without agoraphobia in adults: a network meta-analysis.Cochrane Database Syst Rev. 2016 Apr 13;4(4):CD011004. doi: 10.1002/14651858.CD011004.pub2. Cochrane Database Syst Rev. 2016. PMID: 27071857 Free PMC article.
-
Automated devices for identifying peripheral arterial disease in people with leg ulceration: an evidence synthesis and cost-effectiveness analysis.Health Technol Assess. 2024 Aug;28(37):1-158. doi: 10.3310/TWCG3912. Health Technol Assess. 2024. PMID: 39186036 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

