Post-marketing surveillance of radium-223 chloride in Japanese patients with castration-resistant prostate cancer with bone metastasis-final analysis of 3-year extended follow-up focusing on bone fractures
- PMID: 40779218
- PMCID: PMC12474616
- DOI: 10.1007/s10147-025-02846-7
Post-marketing surveillance of radium-223 chloride in Japanese patients with castration-resistant prostate cancer with bone metastasis-final analysis of 3-year extended follow-up focusing on bone fractures
Abstract
Background: A post-marketing surveillance (PMS) study was conducted in Japan to assess real-world outcomes with radium-223 treatment in men with metastatic castration-resistant prostate cancer (mCRPC). Results from the treatment period showed that radium-223 was generally well tolerated. Follow-up was subsequently extended to 3 years to collect data on fracture events. Results of the extended follow-up are now reported.
Methods: This prospective, non-interventional, multicenter, single-cohort PMS study enrolled men with CRPC and bone metastases treated with radium-223 under clinical practice. Extended follow-up lasted until 3 years after the first administration of radium-223. Data on clinical fractures and survival were collected.
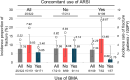
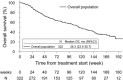
Results: A total of 334 patients were enrolled, with a median follow-up of 15.3 months (range 1-50). The overall incidence proportion of fractures reported as adverse events was 7.76% (95% confidence interval [CI] 5.09-11.25%), with a fracture incidence rate of 5.22 patients [with fracture]/100 person-years (PY). Patients who received bone-modifying agents (BMAs) had a numerically lower incidence of fractures (5.85%; 3.46/100PY vs 9.93%; 7.92/100PY). Median overall survival was 26.32 months (95% CI 21.65-not reached).
Conclusion: Compared with existing reference data, there was no obvious increase in the incidence of clinical fractures in Japanese patients with mCRPC who were treated with radium-223 under clinical practice. As is already well known for androgen deprivation, BMAs may also be useful in reducing bone fracture after radium-223.
Keywords: Bone metastases; Castration-resistant prostate cancer; Japanese patients; Post-marketing surveillance; Radium-223; Real-world data.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: NM received honoraria from Janssen, Astellas, Bayer, Ono Pharmaceutical, and Takeda, and research funding from MSD. ST received honoraria from MSD, Eisai, and Ono Pharmaceutical, and research grants from Eisai, Chugai, Bayer, AstraZeneca, BMS, Ono Pharmaceutical, Daiichi-Sankyo, Novartis, Taiho, and MSD. HU received honoraria from Bayer, Janssen, Takeda, Sanofi and AstraZeneca, and research funding from AstraZeneca. TS, KS, YM, MA, and HK are employees of Bayer Yakuhin, Ltd. MH, YK, and SK reported no conflict of interest. Informed consent: This study was conducted in accordance with Japanese Ministerial Ordinance on Good Post-marketing Study Practice, which does not mandate institutional review board approval for each participating center or written informed consent from patients.
Figures


References
-
- Bubendorf L, Schöpfer A, Wagner U et al (2000) Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol 31(5):578–583 - PubMed
-
- Kirby M, Hirst C, Crawford ED (2011) Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract 65(11):1180–1192 - PubMed
-
- Kurth H, McKiernan J, Bentkover JD et al (2005) Quality of life and pain among prostate cancer patients with bone metastases. J Clin Oncol 23(16 Suppl):4747
-
- Weinfurt KP, Li Y, Castel LD et al (2005) The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol 16(4):579–584 - PubMed
-
- Sathiakumar N, Delzell E, Morrisey MA et al (2011) Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US Medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis 14(2):177–183 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- honoraria/Janssen Japan
- honoraria/Astellas Pharma
- honoraria/Bayer Yakuhin
- research funding/Bayer Yakuhin
- honoraria/Ono Pharmaceutical
- research funding/Ono Pharmaceutical
- honoraria/Takeda Pharmaceutical Company
- research funding/Merck Sharp and Dohme K.K.
- honoraria/Merck Sharp and Dohme K.K.
- honoraria/Eisai
- research funding/Eisai
- research funding/Chugai Pharmaceutical
- research funding/AstraZeneca KK
- honoraria/AstraZeneca KK
- research funding/Bristol-Myers Squibb
- research funding/Daiichi-Sankyo
- research funding/Novartis
- research funding/Taiho Pharmaceutical
- honoraria/Sanofi K.K.
LinkOut - more resources
Full Text Sources
Medical
Research Materials

