Caloric Restriction Enhances the Efficacy of Antiandrogen Therapy in Prostate Cancer by Inhibiting Androgen Receptor Translation
- PMID: 40779415
- PMCID: PMC12535181
- DOI: 10.1158/0008-5472.CAN-24-1986
Caloric Restriction Enhances the Efficacy of Antiandrogen Therapy in Prostate Cancer by Inhibiting Androgen Receptor Translation
Abstract
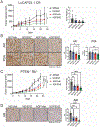
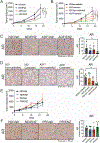
Epidemiologic studies suggest that diet can affect the incidence, progression, and response to treatment in multiple cancers, including prostate cancer. In this study, we investigated the use of dietary interventions, specifically caloric or protein restriction, in combination with antiandrogen therapy as a treatment for prostate cancer. Caloric restriction through alternate-day fasting (ADF) reduced androgen receptor (AR) expression and signaling. This reduction in AR enhanced the antitumor activity of the AR antagonist enzalutamide in multiple mouse models of prostate cancer. Mechanistic studies revealed that nutrient starvation via ADF predominantly decreased AR mRNA translation at the elongation stage due to AA limitation. Pharmacologic agents that similarly impair translation elongation and promote ribosome collisions mimicked the AR translation reduction observed with ADF. Overall, these findings suggest that AA limitation through ADF impairs translation elongation in prostate cancer, to which AR mRNA translation is susceptible, leading to a reduction in AR protein levels and enhancing AR-targeted therapy.
Significance: Fasting-induced caloric restriction reduces androgen receptor translation and enhances the activity of enzalutamide, suggesting that dietary intervention could be an effective strategy to enhance prostate cancer sensitivity to antiandrogen therapy. See related commentary by Anzules et al., p. 4045.
©2025 American Association for Cancer Research.
Conflict of interest statement
Conflict of Interest
The other authors declare no potential conflicts of interest.
Figures


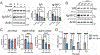


References
MeSH terms
Substances
Grants and funding
- U24 CA274159/CA/NCI NIH HHS/United States
- R35 GM136331/GM/NIGMS NIH HHS/United States
- R01 CA234162/CA/NCI NIH HHS/United States
- R21 CA221942/CA/NCI NIH HHS/United States
- P30 CA082709/CA/NCI NIH HHS/United States
- R21CA221942/National Institutes of Health (NIH)
- GM136331/National Institutes of Health (NIH)
- R01CA234162/National Institutes of Health (NIH)
- U24CA274159/National Institutes of Health (NIH)
- Ralph W. and Grace M. Showalter Research Trust Fund (Showalter Foundation)
- PCF22CHAL13/Prostate Cancer Foundation (PCF)
- HT9425-24-1-0525/U.S. Department of Defense (DOD)
- P30CA082709/National Institutes of Health (NIH)
- IRG-22-147-37-IRG-06/American Cancer Society (ACS)
- Bakewell Foundation (The Bakewell Foundation)
- APP1177797/National Health and Medical Research Council (NHMRC)
- Australian Youth and Health Foundation (Australian Youth Foundation)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

