Expression of human A53T alpha-synuclein without endogenous rat alpha-synuclein fails to elicit Parkinson's disease-related phenotypes in a novel humanized rat model
- PMID: 40779487
- PMCID: PMC12334032
- DOI: 10.1371/journal.pone.0329823
Expression of human A53T alpha-synuclein without endogenous rat alpha-synuclein fails to elicit Parkinson's disease-related phenotypes in a novel humanized rat model
Abstract
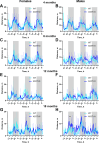
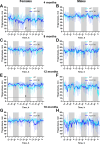
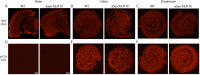
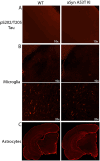
Alpha-synuclein (aSyn) is linked to Parkinson's disease (PD) through SNCA genetic mutations, phosphorylated aSyn in Lewy bodies and Lewy neurites, and most recently through evidence of aSyn aggregation in patient spinal fluid using the aSyn seed amplification assay. Therefore, understanding the biology of this protein and developing therapeutic interventions targeting pathological processing of aSyn are a key area of focus for novel treatments to slow or stop PD. Reliable preclinical models are imperative for these efforts. To this end, we developed a novel model using CRISPR/Cas9 to humanize the regions surrounding the naturally occurring threonine 53 amino acid in the Sprague Dawley rat to generate a humanized A53T aSyn rat model (aSyn A53T KI). We also generated an Snca knockout (aSyn KO) line to pair with the humanized A53T aSyn rat line to confirm that phenotypes were not due to loss of endogenous rat aSyn protein. A systematic phenotyping study was performed on these lines, assessing PD-related pathology and phenotypes at multiple timepoints. The aSyn KO rat line was profiled at 6 and 12 months of age, revealing successful aSyn protein knockout. The aSyn A53T KI model was profiled at 4, 8, 12, and 18 months of age for motor and non-motor phenotypes, nigrostriatal degeneration, and brain pathology. We confirmed the aSyn A53T KI rat expresses human aSyn while lacking endogenous rat aSyn. Motor function and non-motor function remain largely unaffected in this model, and no overt nigrostriatal degeneration or brain pathology are observed up to 18 months of age. Although the aSyn A53T KI rat lacks the ability to model PD pathology and phenotypes at baseline, it is an ideal model for investigating the impact of exogenous synuclein aggregates or environmental triggers on human aSyn in an in vivo model system.
Copyright: © 2025 Polinski et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures









References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

