An energy-embodied paralleled liquid manipulation for equipment-free, quantitative multiplexed liver function monitoring
- PMID: 40779627
- PMCID: PMC12333680
- DOI: 10.1126/sciadv.adx0092
An energy-embodied paralleled liquid manipulation for equipment-free, quantitative multiplexed liver function monitoring
Abstract
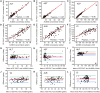
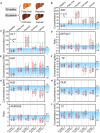
Comprehensive liver function monitoring is vital for liver damage diagnoses. However, multiplexed testing with clinical accuracy often requires paralleled liquid manipulation to avoid reagent interference and nonspecific adsorption, making it restricted to centralized facilities and specialists. Here, we report an equipment-free, quantitative, and multiplexed point-of-care testing (POCT), the energy-embodied POCT (EE-POCT). The driving energy (pressured gas) and liquid reagents are embodied in separate rigid-flexible chambers through a mechanical pressure sensing mechanism to achieve a paralleled, interference-free, and multiple-reagent operation with no need for external equipment. The EE-POCT achieves quantitative results for nine liver-related indicators from 180 microliters of whole blood within 4 minutes. Validated with 103 clinical specimens, it shows high concordance with a clinical gold standard analyzer (Pearson coefficients >0.9). The EE-POCT platform not only advances liver health diagnostics but also sets a precedent for developing versatile, accessible medical technologies that can significantly affect global health outcomes.
Figures






Similar articles
-
Can a Liquid Biopsy Detect Circulating Tumor DNA With Low-passage Whole-genome Sequencing in Patients With a Sarcoma? A Pilot Evaluation.Clin Orthop Relat Res. 2025 Jan 1;483(1):39-48. doi: 10.1097/CORR.0000000000003161. Epub 2024 Jun 21. Clin Orthop Relat Res. 2025. PMID: 38905450
-
Monitoring and optimization of POCT devices in a multi-specialty hospital in Poland: usage trends, quality assurance, and clinical impact (2017-2024).Acta Biochim Pol. 2025 Jun 11;72:14299. doi: 10.3389/abp.2025.14299. eCollection 2025. Acta Biochim Pol. 2025. PMID: 40567349 Free PMC article.
-
[Guidelines for the prevention and management of bronchial asthma (2024 edition)].Zhonghua Jie He He Hu Xi Za Zhi. 2025 Mar 12;48(3):208-248. doi: 10.3760/cma.j.cn112147-20241013-00601. Zhonghua Jie He He Hu Xi Za Zhi. 2025. PMID: 40050074 Chinese.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Biomarkers as point-of-care tests to guide prescription of antibiotics in people with acute respiratory infections in primary care.Cochrane Database Syst Rev. 2022 Oct 17;10(10):CD010130. doi: 10.1002/14651858.CD010130.pub3. Cochrane Database Syst Rev. 2022. PMID: 36250577 Free PMC article.
References
-
- Gao B., Jeong W.-I., Tian Z., Liver: An organ with predominant innate immunity. Hepatology 47, 729–736 (2007). - PubMed
-
- Chalasani N., Younossi Z., Lavine J. E., Diehl A. M., Brunt E. M., Cusi K., Charltion M., Sanyal A. J., American Gastroenterological Association, American Association for the Study of Liver Diseases, American College of Gastroenterologyh , The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 142, 1592–1609 (2012). - PubMed
-
- Xin S., Xu J., Yu Y., Abnormal liver function tests of patients with Coronavirus disease 2019 in mainland China: A systematic review and meta- analysis. J. Gastrointestin. Liver Dis. 29, 219–226 (2020). - PubMed
-
- Devarbhavi H., Asrani S. K., Arab J. P., Nartey Y. A., Pose E., Kamath P. S., Global burden of liver disease: 2023 update. J. Hepatol. 79, 516–537 (2023). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

