Nonlinear spatial integration allows the retina to detect the sign of defocus in natural scenes
- PMID: 40779628
- PMCID: PMC12333688
- DOI: 10.1126/sciadv.adq6320
Nonlinear spatial integration allows the retina to detect the sign of defocus in natural scenes
Abstract
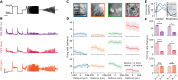
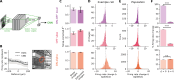
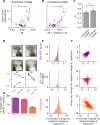
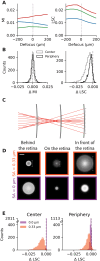
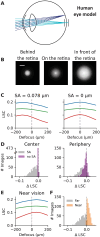
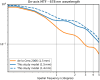
Eye growth is regulated by the visual input. Many studies suggest that the retina can detect whether a visual image is focused in front of or behind the back of the eye and modulate eye growth to bring it back to focus. How can the retina distinguish between these two types of defocus? Here, we simulated how eye optics transforms natural images and recorded how the isolated retina responds to different types of simulated defocus. We found that some ganglion cell types could distinguish between an image focused in front of or behind the retina by estimating spatial contrast. Aberrations in the eye optics made spatial contrast, but not luminance, a reliable cue to distinguish these two types of defocus. Our results suggest a mechanism for how the retina can estimate the sign of defocus and provide an explanation for several results aiming at mitigating strong myopia by slowing down eye growth.
Figures

Similar articles
-
Consequences of eye movements for spatial selectivity.Curr Biol. 2024 Jul 22;34(14):3265-3272.e4. doi: 10.1016/j.cub.2024.06.016. Epub 2024 Jul 8. Curr Biol. 2024. PMID: 38981478 Free PMC article.
-
Laser-assisted subepithelial keratectomy (LASEK) versus laser-assisted in-situ keratomileusis (LASIK) for correcting myopia.Cochrane Database Syst Rev. 2017 Feb 15;2(2):CD011080. doi: 10.1002/14651858.CD011080.pub2. Cochrane Database Syst Rev. 2017. PMID: 28197998 Free PMC article.
-
Model eye evaluation of new extended depth of focus designs versus defocus-based lenslet designs for myopia management.Optom Vis Sci. 2025 Aug 1;102(8):513-525. doi: 10.1097/OPX.0000000000002269. Epub 2025 Jun 3. Optom Vis Sci. 2025. PMID: 40460196
-
Temporal color contrast guides emmetropization in chick.Exp Eye Res. 2021 Jan;202:108331. doi: 10.1016/j.exer.2020.108331. Epub 2020 Nov 3. Exp Eye Res. 2021. PMID: 33152390
-
Excimer laser refractive surgery versus phakic intraocular lenses for the correction of moderate to high myopia.Cochrane Database Syst Rev. 2014 Jun 17;2014(6):CD007679. doi: 10.1002/14651858.CD007679.pub4. Cochrane Database Syst Rev. 2014. PMID: 24937100 Free PMC article.
References
-
- Kruger P. B., Mathews S., Aggarwala K. R., Sanchez N., Chromatic aberration and ocular focus: Fincham revisited. Vision Res. 33, 1397–1411 (1993). - PubMed
-
- Wilson B. J., Decker K. E., Roorda A., Monochromatic aberrations provide an odd-error cue to focus direction. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 19, 833–839 (2002). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

