A two-step mechanism for RIG-I activation by influenza virus mvRNAs
- PMID: 40779639
- PMCID: PMC12333679
- DOI: 10.1126/sciadv.adw8034
A two-step mechanism for RIG-I activation by influenza virus mvRNAs
Abstract
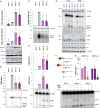
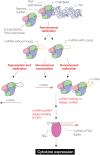
Influenza A virus (IAV) noncanonical RNAs are bound by retinoic acid-inducible gene I (RIG-I). However, innate immune activation is infrequent and it is not understood why noncanonical IAV RNAs activate RIG-I in a sequence- or RNA structure-dependent manner. We hypothesized that multiple events need to occur before IAV RNA synthesis activates RIG-I and investigated whether RIG-I activation is stimulated by the noncanonical or aberrant transcription of mini viral RNAs (mvRNA), an RNA that is overexpressed in highly pathogenic IAV infections. We find that mvRNAs can cause noncanonical transcription termination through a truncated 5' polyadenylation signal or a 5' transient RNA structure that interrupts polyadenylation. The resulting capped complementary RNAs stimulate the release of an mvRNA and complement RIG-I activation in trans. Overall, our findings indicate that sequential rounds of noncanonical or aberrant viral replication and transcription are needed for innate immune signaling by IAV RNA synthesis.
Figures




References
-
- de Jong M. D., Simmons C. P., Thanh T. T., Hien V. M., Smith G. J., Chau T. N., Hoang D. M., Chau N. V., Khanh T. H., Dong V. C., Qui P. T., Cam B. V., Ha D. Q., Guan Y., Peiris J. S., Chinh N. T., Hien T. T., Farrar J., Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 12, 1203–1207 (2006). - PMC - PubMed
-
- de Wit E., Siegers J. Y., Cronin J. M., Weatherman S., van den Brand J. M., Leijten L. M., van Run P., Begeman L., van den Ham H. J., Andeweg A. C., Bushmaker T., Scott D. P., Saturday G., Munster V. J., Feldmann H., van Riel D., 1918 H1N1 influenza virus replicates and induces proinflammatory cytokine responses in extrarespiratory tissues of ferrets. J. Infect. Dis. 217, 1237–1246 (2018). - PMC - PubMed
-
- Kash J. C., Tumpey T. M., Proll S. C., Carter V., Perwitasari O., Thomas M. J., Basler C. F., Palese P., Taubenberger J. K., Garcia-Sastre A., Swayne D. E., Katze M. G., Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature 443, 578–581 (2006). - PMC - PubMed
-
- Te Velthuis A. J. W., Long J. C., Bauer D. L. V., Fan R. L. Y., Yen H. L., Sharps J., Siegers J. Y., Killip M. J., French H., Oliva-Martin M. J., Randall R. E., de Wit E., van Riel D., Poon L. L. M., Fodor E., Mini viral RNAs act as innate immune agonists during influenza virus infection. Nat. Microbiol. 3, 1234–1242 (2018). - PMC - PubMed
-
- Kobasa D., Jones S. M., Shinya K., Kash J. C., Copps J., Ebihara H., Hatta Y., Kim J. H., Halfmann P., Hatta M., Feldmann F., Alimonti J. B., Fernando L., Li Y., Katze M. G., Feldmann H., Kawaoka Y., Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature 445, 319–323 (2007). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

