Dual Immune Check Point Blockade in MGMT-Unmethylated Newly Diagnosed Glioblastoma: NRG Oncology BN007, a Randomized Phase II/III Clinical Trial
- PMID: 40779733
- PMCID: PMC12440284
- DOI: 10.1200/JCO-25-00618
Dual Immune Check Point Blockade in MGMT-Unmethylated Newly Diagnosed Glioblastoma: NRG Oncology BN007, a Randomized Phase II/III Clinical Trial
Abstract
Purpose: New therapies for glioblastoma are needed, especially MGMT-unmethylated (uMGMT) disease. NRG Oncology BN002 (phase I) demonstrated safety and suggested efficacy of ipilimumab (ipi) with nivolumab (nivo) in newly diagnosed glioblastoma, leading to this phase II/III trial.
Methods: Adults with newly diagnosed uMGMT glioblastoma and Karnofsky performance status (KPS) ≥70 were randomly assigned to radiotherapy with either immunotherapy (ipi and nivo) or temozolomide (TMZ), stratified by recursive partitioning analysis (RPA) class and intention to use tumor treating fields. With 95% power to detect a hazard ratio (HR) ≤0.58 for progression-free survival (PFS) at a one-sided significance level (P) of .15, superior PFS with immunotherapy in phase II would lead to phase III overall survival (OS) testing. Corticosteroids were disallowed when starting immunotherapy. Diagnosis, biomarkers, and PFS were centrally assessed.
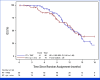
Results: One hundred fifty-nine participants were randomly assigned (79 immunotherapy and 80 TMZ). Arms were well balanced for age (median 60 years, range, 28-79), sex (male n = 105, 66%), KPS (90-100 n = 97, 61%), resection extent (gross total, n = 103, 65%), and RPA class (III, n = 16, 10%; IV, n = 116, 73%; V, n = 27, 17%). A preplanned analysis of phase II data conducted after 100 centrally determined PFS events showed no significant PFS improvement for ipi and nivo versus TMZ (median 7.7 months v 8.5 months, HR, 1.47 [70% CI, 1.19 to 1.83]; one-sided P = .96 [95% CI, 0.98 to 2.2]). OS is immature (>50% alive) but with no observed difference between arms (median approximately 13 months each, HR, 0.95 [95% CI, 0.61 to 1.49]; P = .36).
Conclusion: Ipi and nivo did not improve PFS among patients with newly diagnosed uMGMT glioblastoma versus TMZ. Accrual closed permanently; the trial will not proceed to phase III. No new safety signals were identified. Molecular correlative analyses and survival follow-up are ongoing.
Trial registration: ClinicalTrials.gov NCT04396860.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

