Effect of arginine supplementation on liver and pectoral muscle: Tissue-specific lipid metabolism in broilers
- PMID: 40779811
- PMCID: PMC12355073
- DOI: 10.1016/j.psj.2025.105601
Effect of arginine supplementation on liver and pectoral muscle: Tissue-specific lipid metabolism in broilers
Abstract
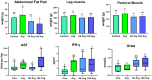
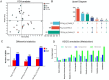
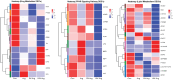
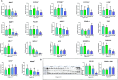
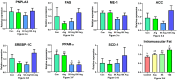
Abdominal fat (AF) and intramuscular fat (IMF) are key carcass traits in broilers but managing both is challenging due to their contrasting effects. Arginine (Arg) supplementation has potential effect in lipid metabolism, however its tissue specific effect remains poorly understood. The objective of this study was to investigate the tissue specific effect of Arg supplementation on growth performance and fat metabolism in both liver and pectoral muscle in broilers. A total of 480 Arbor Acre chicks were randomly assigned to four groups: Control (0 g/kg), Arg (1.8 g/kg), 5 × Arg (9 g/kg) and 10 × Arg (18 g/kg), with 12 replicates of 10 birds each. Overall, high Arg supplementation (5 ×, 10 ×) significantly impaired growth performance, reducing average daily gain and feed intake, accompanied by elevated serum AST and IFN-γ levels (P < 0.05). Liver transcriptomics analysis revealed that 10 × Arg significantly enriched PPAR signaling pathway, promoting fatty acid oxidation while suppressing lipogenic genes. Conversely, in pectoral muscle, high Arg (10 ×) promoted intramuscular fat deposition which was associated with downregulation of PPAR-α (P < 0.05) and increased expression of key lipogenic genes involved in de novo lipogenesis (SREBP-1c, FAS, ACC and SCD). Moreover, Arg supplementation modulated drug metabolism genes in liver, including EPX and RRM2, suggesting potential impacts on detoxification pathways. These findings underscore the importance of precise Arg dosing to optimize broiler growth, immune function, and carcass quality by targeting its tissue specific metabolic effect.
Keywords: Abdominal fat; Arginine; Broilers; Intramuscular fat; Lipogenic genes.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Disclosures All authors read and approved the final manuscript. And the authors declare no conflict of interest in this study.
Figures

Similar articles
-
A study of solely used phytase or in combination with multi-carbohydrase on growth performance along with tibia mineralization, and carcass traits in broilers fed nutrient-deficient diets.J Anim Sci. 2024 Jan 3;102:skae299. doi: 10.1093/jas/skae299. J Anim Sci. 2024. PMID: 39367522
-
The Effects of Different Level of Synbiotic Supplementation in Diet of Broiler on Growth Performance, Intestinal Histology and Microbial Colony.Arch Razi Inst. 2024 Dec 31;79(6):1227-1234. doi: 10.32592/ARI.2024.79.6.1227. eCollection 2024 Dec. Arch Razi Inst. 2024. PMID: 40599451 Free PMC article.
-
Enhancing skeletal muscle fiber characteristics, intramuscular fat deposition, and fatty acid composition in broilers under heat stress through combined selenomethionine and Bacillus subtilis supplementation in the diet.J Anim Sci. 2024 Jan 3;102:skae267. doi: 10.1093/jas/skae267. J Anim Sci. 2024. PMID: 39301922
-
Research advances in intramuscular fat deposition and chicken meat quality: genetics and nutrition.J Anim Sci Biotechnol. 2025 Jul 16;16(1):100. doi: 10.1186/s40104-025-01234-5. J Anim Sci Biotechnol. 2025. PMID: 40665461 Free PMC article. Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Abdullah H.M., Bielke L.R., Helmy Y.A. Effect of arginine supplementation on growth performance and immunity of broilers: a review. J. Glob. Innov. Agric. Sci. 2019;7:141–144.
-
- Ali I.K., Zaed J.M.S. Effect of adding L-Arginine to broiler diets on physiological performance. Proc. IOP Conf. Ser.: Earth Environ. Sci. 2023;1262
-
- Al-Zharani M., Mubarak M., Rudayni H.A., Abdelwahab M.M., Al-Eissa M. Intoxication induced by urea containing diets in broiler chickens: effect on weight gain, feed conversion ratio, hematological and biochemical profiles. Adv. Biosci. Biotechnol. 2023;14:106–119. doi: 10.4236/abb.2023.143007. - DOI
-
- Arbor Acres. 2022. Arbor acre broiler nutrient specification.
-
- Bougarne N., Weyers B., Desmet S.J., Deckers J., Ray D.W., Staels B., De Bosscher K. Molecular actions of PPAR α in lipid metabolism and inflammation. Endocr. Rev. 2018;39:760–802. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

