Oxidative modulation of Piezo1 channels
- PMID: 40779836
- PMCID: PMC12355560
- DOI: 10.1016/j.redox.2025.103797
Oxidative modulation of Piezo1 channels
Abstract
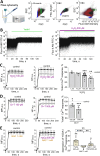
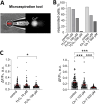
Emerging evidence suggests that mechanosensitive Piezo1 channels play a role in the pathomechanism of various disorders. However, the mechanisms by which accumulating pathologies regulate Piezo1 activation remain unclear. Oxidative stress, a common feature of neurodegenerative diseases, is associated with generation of reactive oxygen species (ROS). While the dependence of Piezo1 channels on temperature, pH, and voltage has been well studied, the redox regulation of these highly mechanosensitive channels remains unknown. We investigated whether oxidative stress modulates the calcium permeability of Piezo1 channels using red blood cells (RBCs) and HEK293T cells transduced with Piezo1 as model systems. Additionally, using the selective H2O2 sensor HyPer7, we examined whether Piezo1 activation induces the generation of endogenous ROS. Using flow cytometry, Ca2+-imaging, patch clamp and microaspiration techniques we demonstrate that cell-permeable oxidants hydrogen peroxide (H2O2) and Chloramine-T, which specifically oxidize cysteines and methionines, inhibited Yoda1-induced activation of Piezo1 in both cell types. In contrast to Chloramine-T, the membrane-impermeable, cysteine-specific oxidant DTNB (5,5'-dithiobis-(2-nitrobenzoic acid)) also inhibited Piezo1, although its inhibitory effect was less pronounced. Mechanical sensitivity of Piezo1 was reduced by H2O2 also in RBCs. Scavenging antioxidants N-acetylcysteine and dithiothreitol decreased or eliminated the inhibitory action of H2O2 and Chloramine-T. However, overexpression of the antioxidant transcription factor Nrf2 (Nuclear factor erythroid 2-related factor 2) did not prevent the inhibitory effects of Chloramine-T, suggesting a membrane-delimited site of redox modulation. Notably, Piezo1 activation slightly increased endogenous H2O2 production. Our data suggest that the reduced activity of Piezo1 in the oxidative environment is determined by oxidation of both cysteines and methionines, which are enriched in intracellular domains, with methionines playing a predominant role. Given the role of Piezo1 channels in pathophysiology of numerous disorders, we propose that, under conditions associated with oxidative stress, redox modulation of these mechanosensors could be a significant factor contributing to disease pathology.
Keywords: Hydrogen peroxide; Mechanoreceptors; Oxidation; Oxidative stress; Piezo1 channel.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/persoslgnal relationships which may be considered as potential competing interests: Tarja Malm reports financial support was provided by Research Council of Finland. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Evtugina N.G., Peshkova A.D., Khabirova A.I., Andrianova I.A., Abdullayeva S., Ayombil F., Shepeliuk T., Grishchuk E.L., Ataullakhanov F.I., Litvinov R.I., Weisel J.W. Activation of Piezo1 channels in compressed red blood cells augments platelet-driven contraction of blood clots. J. Thromb. Haemostasis. 2023;21:2418–2429. doi: 10.1016/j.jtha.2023.05.022. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

