Nuclear lamina phase separation orchestrates stress-induced transcriptional responses in plants
- PMID: 40780209
- PMCID: PMC12338065
- DOI: 10.1016/j.devcel.2025.07.008
Nuclear lamina phase separation orchestrates stress-induced transcriptional responses in plants
Abstract
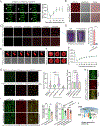
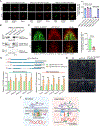
The nuclear lamina (NL), a perinuclear protein meshwork formed by nucleoskeleton and inner nuclear membrane (INM) proteins, is crucial for chromatin organization at the nuclear periphery and gene expression regulation in eukaryotic cells. However, NL-dependent transcriptional regulation remains poorly understood in plants due to the absence of most canonical NL proteins found in animals. Here, we report that the plant INM protein PLANT NUCLEAR ENVELOPE TRANSMEMBRANE 2 (PNET2) interacts with membrane-bound NAC (NAM, ATAF1/2, and CUC2) transcription factors, NTLs, via intrinsic disorder regions and promotes liquid-liquid phase separation within the NL. This compartmentalization effectively sequesters NTLs and restricts their transcriptional activity. In the absence of PNET2, NTLs become deregulated, triggering spontaneous and broad-spectrum stress responses. Importantly, we found that stress stimuli, such as heat shock, disrupt PNET2-NTL phase separation, releasing NTLs for target gene binding and transcriptional activation. These findings demonstrate a phase separation-based regulatory mechanism within the NL that controls membrane-bound transcription factor activity in response to environmental cues.
Keywords: NTL; PNET2; gene expression regulations; membrane-bound transcription factors; nuclear lamina; nuclear membrane; nucleoskeleton; phase separation; plant cells; stress responses.
Copyright © 2025 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


Similar articles
-
Phase separation as a key mechanism in plant development, environmental adaptation, and abiotic stress response.J Biol Chem. 2025 Jun;301(6):108548. doi: 10.1016/j.jbc.2025.108548. Epub 2025 Apr 24. J Biol Chem. 2025. PMID: 40286852 Free PMC article. Review.
-
T-bet expressing Tr1 cells driven by dietary signals dominate the small intestinal immune landscape.bioRxiv [Preprint]. 2025 Jul 4:2025.06.30.662190. doi: 10.1101/2025.06.30.662190. bioRxiv. 2025. PMID: 40747421 Free PMC article. Preprint.
-
The Heat Shock Transcription Factor HsfA Plays a Role in Membrane Lipids Biosynthesis Connecting Thermotolerance and Unsaturated Fatty Acid Metabolism in Aspergillus fumigatus.Microbiol Spectr. 2023 Jun 15;11(3):e0162723. doi: 10.1128/spectrum.01627-23. Epub 2023 May 17. Microbiol Spectr. 2023. PMID: 37195179 Free PMC article.
-
NFATc1 marks articular cartilage progenitors and negatively determines articular chondrocyte differentiation.Elife. 2023 Feb 15;12:e81569. doi: 10.7554/eLife.81569. Elife. 2023. PMID: 36790146 Free PMC article.
-
From gene to mechanics: a comprehensive insight into the mechanobiology of LMNA mutations in cardiomyopathy.Cell Commun Signal. 2024 Mar 27;22(1):197. doi: 10.1186/s12964-024-01546-5. Cell Commun Signal. 2024. PMID: 38539233 Free PMC article. Review.
References
-
- Gaillard MC, and Reddy KL (2018). The Nuclear Lamina and Genome Organization. In Nuclear Architecture and Dynamics, Lavelle C, and Victor J-M, eds. (Academic Press; ), pp. 321–343. 10.1016/B978-0-12-803480-4.00014-4. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

