Effectiveness and safety of the recombinant zoster vaccine in adult patients with systemic lupus erythematosus: a claims-based retrospective cohort study in the USA
- PMID: 40780731
- PMCID: PMC12336505
- DOI: 10.1136/rmdopen-2025-005839
Effectiveness and safety of the recombinant zoster vaccine in adult patients with systemic lupus erythematosus: a claims-based retrospective cohort study in the USA
Abstract
Objectives: To assess vaccine effectiveness (VE) and safety of the recombinant zoster vaccine (RZV) in adults with systemic lupus erythematosus (SLE).
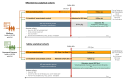
Methods: This retrospective study using administrative claims data collected between 1 January 2018 and 30 September 2023 from the USA included two cohorts of adults aged ≥18 years with SLE insured by (1) Medicare (parts A/B/D) or (2) one of five commercial partners (including Medicare Advantage plans). In VE analyses, adults receiving two RZV doses ≥28 days apart were matched 1:4 to unvaccinated comparators on insurer, sex and age (±5 years); the outcome was incident herpes zoster (HZ) after 31 days. In safety analyses, adults receiving RZV dose 1 or 2 were matched to unvaccinated comparators as above; the outcome was severe SLE flare within 90 days. Adjusted hazard ratios (HRs) were estimated using Cox models with inverse probability of treatment weighting (IPTW).
Results: VE cohorts included 2284 Medicare and 1308 commercially insured RZV-vaccinated patients; mean weighted follow-up was 1.4-1.6 person-years. Safety cohorts included 6602 RZV vaccinations (dose 1 or 2) in Medicare and 4196 in commercial insurers. Vaccinated versus unvaccinated patient characteristics were balanced after IPTW. VE was 70% (95% CI: 50% to 82%) in Medicare and 54% (95% CI: 18% to 74%) in commercially insured patients. The HR of severe SLE flare in vaccinated versus unvaccinated patients was 0.91 (95% CI: 0.75 to 1.11) in Medicare and 0.94 (95% CI: 0.72 to 1.24) in commercially insured patients.
Conclusions: RZV prevented a majority of HZ cases in individuals with SLE without increasing the risk of severe SLE flare.
Keywords: Epidemiology; Lupus Erythematosus, Systemic; Vaccination.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: OS, DO, OA, HS and HY are employees of GSK and hold financial equities in GSK. DAD is an employee of CVS Health and owns stock in CVS Health. CNM-W is a former employee of CVS Health and owns stock in CVS Health. KD reports research contracts through Carelon Research with Pfizer, AbbVie, AstraZeneca and GSK. RPO and NZ are employees of Optum and own stock in UnitedHealth Group. KP reports research contracts with GSK, AbbVie, Pfizer and Sanofi. AO reports financial relationships with AbbVie, Amgen, Bristol Myers Squibb, Celgene, CorEvitas, Forward/National Databank for Rheumatic Diseases, Gilead, GSK, Janssen, Lilly, NIH/NIAMS, Novartis, Pfizer, Rheum Research Foundation, Takeda, TREG and UCB. MDG reports financial relationships as adviser/consultant with Pfizer and AbbVie, and receiving grants/research support from GSK, Janssen and Pfizer, SEM, SAK, ALS, JSK, CH, MH, KS, AJ-A, QM and MS have nothing to disclose.
Figures

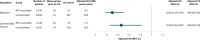

References
-
- Centers for Disease Control and Prevention Clinical overview of herpes zoster (shingles) 2024. https://www.cdc.gov/shingles/hcp/clinical-overview/index.html Available.
-
- Centers for Disease Control and Prevention Clinical features of shingles (herpes zoster) 2024. https://www.cdc.gov/shingles/hcp/clinical-signs/index.html Available.
-
- Centers for Disease Control and Prevention Shingles facts and stats. 2024. https://www.cdc.gov/shingles/data-research/?CDC_AAref_Val=https://www.cd... Available.
-
- Furer V, Rondaan C, Heijstek M, et al. Incidence and prevalence of vaccine preventable infections in adult patients with autoimmune inflammatory rheumatic diseases (AIIRD): a systemic literature review informing the 2019 update of the EULAR recommendations for vaccination in adult patients with AIIRD. RMD Open. 2019;5:e001041. doi: 10.1136/rmdopen-2019-001041. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
