Early Locus Coeruleus noradrenergic axon loss drives olfactory dysfunction in Alzheimer's disease
- PMID: 40781079
- PMCID: PMC12334674
- DOI: 10.1038/s41467-025-62500-8
Early Locus Coeruleus noradrenergic axon loss drives olfactory dysfunction in Alzheimer's disease
Abstract
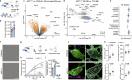
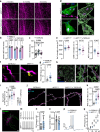
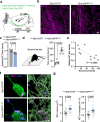
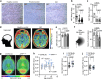
Alzheimer's disease (AD) often begins with non-cognitive symptoms such as olfactory deficits, which can predict later cognitive decline, though the mechanisms remain unclear. Pathologically, the brainstem locus coeruleus (LC), the main source of the neurotransmitter noradrenalin (NA) modulating olfactory information processing is affected early. Here we show early and distinct loss of noradrenergic input to the olfactory bulb (OB) coinciding with impaired olfaction in an AD mouse model, before appearance of amyloid plaques. Mechanistically, OB microglia recognize and phagocytose LC axons. Reducing phagocytosis genetically preserves LC axons and olfaction. Prodromal AD patients display elevated TSPO-PET signals in the OB, similarly to AppNL-G-F mice. We further confirm early LC axon degeneration in post-mortem OBs in patients with early AD. Our findings reveal a mechanism linking early LC damage to hyposmia in AD, suggesting olfactory testing and neurocircuit imaging for early diagnosis and enable timely therapeutic intervention for Alzheimer's disease.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.B. received consulting/speaker honoraria from Life Molecular Imaging, GE Healthcare, and Roche, and reader honoraria from Life Molecular Imaging. All other authors declare no competing interests.
Figures


References
-
- Report, A. A. 2023 Alzheimer’s disease facts and figures. Alzheimer’s Dement.19, 1598–1695 (2023). - PubMed
-
- Reardon, S. Alzheimer’s drug donanemab: what promising trial means for treatments. Nature617, 232–233 (2023). - PubMed
-
- Braak, H., Thal, D. R., Ghebremedhin, E. & Tredici, K. D. Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol.70, 960–969 (2011). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

