Innovative diagnostic approaches for lung cancer: integrating traditional cytology with qPCR for rapid and reliable results
- PMID: 40781107
- PMCID: PMC12334670
- DOI: 10.1038/s41598-025-13743-4
Innovative diagnostic approaches for lung cancer: integrating traditional cytology with qPCR for rapid and reliable results
Abstract
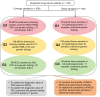
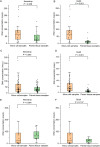
To address inherent limitations of standard diagnostic procedures for lung cancer, like long turn-around-time and the need for sufficient samples for analysis, we innovatively integrated traditional smear cytology (TSC) with quantitative polymerase chain reaction (qPCR) assays on micro cell samples (MCSs) for the diagnosis of lung cancer. All patients underwent TSC and qPCR assays targeting 11 genes based on different MCSs, including samples obtained by flushing needles used for endobronchial ultrasound biopsies and percutaneous aspiration biopsies of lung in 38 cases (G1) and 108 cases (G2), and lavage fluid samples obtained by bronchoalveolar lavage in 38 cases (G3). With clinical diagnosis and pathological biopsy as diagnostic gold standard, the diagnostic value of these MCSs were explored. In G1, G2 and G3, the diagnostic yield of MCSs-based TSC alone was 78.9%, 93.5% and 76.3%, respectively. Combination of TSC and genetic testing increased the diagnostic yield to 81.6%, 98.1% and 84.2% in G1, G2 and G3, respectively. In addition, the qPCR results of MCSs and paired tissue samples was all matched with a concordance rate of 100%, and the quantity of DNA extracted from our samples was significantly higher than that of tissue samples in G1 and G2. Notably, the TAT of our diagnostic method required only 24 h, which greatly improved the timeliness of diagnosis. Our study demonstrated that MCSs-based TSC combined with genetic testing could not only rapidly and reliably diagnose lung cancer, but also effectively detect gene targets, with potential for widespread application.
Keywords: Diagnosis; Lung cancer; QPCR; Traditional smear cytology; Turn-around-time.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical statement: The study was carried out based on the Declaration of Helsinki and approved by the Ethics Committee of Henan Cancer Hospital Affiliated Cancer Hospital of Zhengzhou University (2023-KY-0065).
Figures


References
-
- Nasim, F., Sabath, B. F. & Eapen, G. A. Lung cancer. Med. Clin. North. Am.103 (3), 463–473 (2019). - PubMed
-
- Han, Y. & Li, J. Sample types applied for molecular diagnosis of therapeutic management of advanced non-small cell lung cancer in the precision medicine. Clin. Chem. Lab. Med.55 (12), 1817–1833 (2017). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

