Pupil size modulation drives retinal activity in mice and shapes human perception
- PMID: 40781243
- PMCID: PMC12334691
- DOI: 10.1038/s41467-025-62736-4
Pupil size modulation drives retinal activity in mice and shapes human perception
Abstract
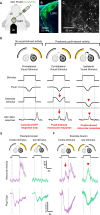
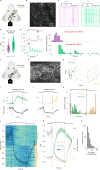
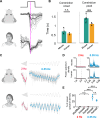
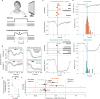
Retinal adaptation is assisted by the pupil, with pupil contraction and dilation thought to prevent global light changes from triggering neuronal activity in the retina. However, we find that pupillary constriction from increased light, the pupillary light reflex (PLR), can drive strong responses in retinal ganglion cells (RGCs) in vivo in mice. The PLR drives neural activity in all RGC types, and pupil-driven activity is relayed to the visual cortex. Furthermore, the consensual PLR allows one eye to respond to luminance changes presented to the other eye, leading to a binocular response and modulation during low-amplitude luminance changes. To test if pupil-induced activity is consciously perceived, we performed psychophysics on human volunteers, finding a perceptual dimming consistent with PLR-induced responses in mice. Our findings thus uncover that pupillary dynamics can directly induce visual activity that is consciously detectable, suggesting an active role for the pupil in encoding perceived ambient luminance.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Erisken, S. et al. Effects of locomotion extend throughout the mouse early visual system. Curr. Biol.24, 2899–2907 (2014). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

