A novel EDA variant that causes X-linked hypohidrotic ectodermal dysplasia in a Chinese family
- PMID: 40781288
- PMCID: PMC12333194
- DOI: 10.1186/s12884-025-07963-9
A novel EDA variant that causes X-linked hypohidrotic ectodermal dysplasia in a Chinese family
Abstract
Background: Hypohidrotic ectodermal dysplasia (HED) is a rare genetic disorder that affects the development of the skin, hair, nails, teeth, and sweat glands. Due to its rarity, there are currently few methods that can be applied to facilitate its diagnosis during the prenatal period. Although a prenatal ultrasonographic examination will detect early signs of the disease, there are few reports on specific prenatal ultrasonographic features of ectodermal dysplasia. Genetic diagnosis can confirm the disease, but the numerous gene variants that cause ectodermal dysplasia have not been fully identified.
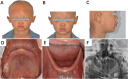
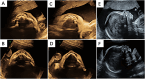
Case presentation: Our case was a multiparous woman carrying a single male fetus who underwent a fetal ultrasound examination at 23 weeks of gestation. The examination revealed thin alveolar bone, with no presence of hypoechoic tooth germ tissue in both the upper and lower alveolar bones. The seven-year-old male proband in this family manifested a clinical phenotype of sparse hair and underdeveloped teeth, and trio-based whole-exome sequencing (WES) performed on both parents and the proband revealed a novel and likely pathogenic variant of the EDA gene (NM_001399.4: c.806G > T, p.Gly269Val) associated with X-linked HED (XLHED; OMIM:305100). Based on the results of the fetal ultrasound examination and the results of the proband's genetic testing, the couple ultimately decided to terminate the pregnancy. The DNA of the fetal skin tissue after the induced abortion was extracted for Sanger sequencing, and it was confirmed that the fetus possessed ectodermal dysplasia generated by the afore-mentioned EDA gene mutation.
Conclusions: Our study suggested that prenatal ultrasonography constituted an effective method for screening ectodermal dysplasia during pregnancy. In addition, our findings expanded the range of EDA variants in XLHED patients; and this discovery may now assist potential patients in receiving an accurate diagnosis, allowing them to make appropriate reproductive decisions.
Keywords: EDA c.806G > T; Hypohidrotic ectodermal dysplasia; Prenatal ultrasound screening; Whole-exome sequencing.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Written informed consent was obtained from the patient and her husband. The study was approved by the Ethics Committee of the First People’s Hospital of Yunnan Province (KHLL2021-169). Consent for publication: We have obtained the consent of all participants with personal information involved in the case and signed the consent for publication. A copy of the signed, written informed consent for publication form is available for review by the editor. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Hypohidrotic Ectodermal Dysplasia.2003 Apr 28 [updated 2025 Mar 20]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2003 Apr 28 [updated 2025 Mar 20]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301291 Free Books & Documents. Review.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Compound heterozygous WNT10A missense variations exacerbated the tooth agenesis caused by hypohidrotic ectodermal dysplasia.BMC Oral Health. 2024 Jan 27;24(1):136. doi: 10.1186/s12903-024-03888-5. BMC Oral Health. 2024. PMID: 38280992 Free PMC article.
-
Beckwith-Wiedemann Syndrome.2000 Mar 3 [updated 2023 Sep 21]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2000 Mar 3 [updated 2023 Sep 21]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301568 Free Books & Documents. Review.
-
Hemophilia A.2000 Sep 21 [updated 2025 Aug 7]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2000 Sep 21 [updated 2025 Aug 7]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301578 Free Books & Documents. Review.
References
-
- Monreal AW, Ferguson BM, Headon DJ, Street SL, Overbeek PA, Zonana J. Mutations in the human homologue of mouse Dl cause autosomal recessive and dominant hypohidrotic ectodermal dysplasia. Nat Genet. 1999;22(4):366–9. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- KHBS-2022-016/Doctor Foundation of the First People's Hospital of Yunnan province.
- 202201AY070001-226/Yunnan Provincial Department of Science and Technology - Kunming Medical University Joint Special Project on Applied Basic Research.
- 82260645/National Natural Area Foundation Project: Study on the association and role of key intestinal flora and its metabolites with the risk of preeclampsia based on birth cohort.
LinkOut - more resources
Full Text Sources

