Maternal phthalate exposure, gestational length, and preterm birth risk: a prospective cohort study nested within a randomised trial
- PMID: 40781296
- PMCID: PMC12335031
- DOI: 10.1186/s12884-025-07980-8
Maternal phthalate exposure, gestational length, and preterm birth risk: a prospective cohort study nested within a randomised trial
Abstract
Background: Preterm birth (< 37 weeks gestation) is a leading cause of infant morbidity and mortality, yet the underlying causes remain unknown in many cases. Environmental exposures, including endocrine-disrupting chemicals such as phthalates, have been implicated in preterm birth risk. Phthalates are commonly used as plasticisers in consumer products, resulting in widespread human exposure. While some studies suggest an association between maternal phthalate exposure and reduced gestational length, findings remain inconsistent. This study aimed to investigate the relationship between urinary phthalate metabolite concentrations and gestational length in an Australian pregnancy cohort.
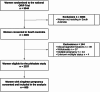
Methods: This prospective cohort study was nested within the Omega-3 to Reduce the Incidence of Prematurity (ORIP) trial. A total of 605 women with singleton pregnancies from South Australia provided urine samples between 22- and 26-weeks' gestation for phthalate metabolite analysis. Thirteen phthalate metabolites were quantified using liquid chromatography-tandem mass spectrometry. Gestational age at birth was determined from medical records. Linear regression models assessed associations between phthalate concentrations and gestational length, adjusting for maternal characteristics including age, BMI, socioeconomic status, education, smoking, and alcohol consumption.
Results: Phthalate metabolites were detected in > 99% of urine samples, with the highest concentrations observed for mono-ethyl phthalate (MEP), mono-isobutyl phthalate (MiBP), and mono-butyl phthalate (MBP). There was no evidence of an association between phthalate exposure and gestational length in either unadjusted or adjusted analyses. No significant association was found between phthalate exposure and preterm birth risk.
Conclusions: Despite widespread phthalate exposure, no clear link was identified between maternal phthalate levels and shortened gestation in this Australian cohort. However, continued surveillance is needed to monitor emerging plasticiser exposures and inform public health policies on maternal and infant health.
Trial registration number: Australian New Zealand Clinical Trials Registry number, ACTRN12613001142729. Date of registration: 27/09/2013.
Keywords: Endocrine disrupting chemicals; Environment; Exposure; Phthalates; Pregnancy; Prematurity; Prenatal.
© 2025. Crown.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethics approval for this study was granted by the Women’s and Children’s Health Network Human Research Ethics Committee (HREC/16/WCHN/80). All participating women provided informed consent prior to inclusion in the study. The study adhered to the principles of the Declaration of Helsinki and complied with all applicable national guidelines and regulations. Consent for publication: Not applicable. This manuscript does not contain any individual person’s data in any form (including individual details, images, or videos). Competing interests: MM was President of the International Society for the Study of Fatty Acids and Lipids (ISSFAL), 2021–2024. No payments have been received in relation to this role. All other authors report no competing interests.
Figures
Similar articles
-
Planned birth at or near term for improving health outcomes for pregnant women with gestational diabetes and their infants.Cochrane Database Syst Rev. 2018 Jan 5;1(1):CD012910. doi: 10.1002/14651858.CD012910. Cochrane Database Syst Rev. 2018. PMID: 29303230 Free PMC article.
-
Techniques of monitoring blood glucose during pregnancy for women with pre-existing diabetes.Cochrane Database Syst Rev. 2017 Jun 11;6(6):CD009613. doi: 10.1002/14651858.CD009613.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2019 May 23;5:CD009613. doi: 10.1002/14651858.CD009613.pub4. PMID: 28602020 Free PMC article. Updated.
-
Use of biochemical tests of placental function for improving pregnancy outcome.Cochrane Database Syst Rev. 2015 Nov 25;2015(11):CD011202. doi: 10.1002/14651858.CD011202.pub2. Cochrane Database Syst Rev. 2015. PMID: 26602956 Free PMC article.
-
Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks' gestation for improving pregnancy outcome.Cochrane Database Syst Rev. 2017 Mar 3;3(3):CD004735. doi: 10.1002/14651858.CD004735.pub4. Cochrane Database Syst Rev. 2017. PMID: 28257562 Free PMC article.
-
Prenatal administration of progestogens for preventing spontaneous preterm birth in women with a multiple pregnancy.Cochrane Database Syst Rev. 2019 Nov 20;2019(11):CD012024. doi: 10.1002/14651858.CD012024.pub3. Cochrane Database Syst Rev. 2019. PMID: 31745984 Free PMC article.
References
-
- Ohuma EO, Moller A-B, Bradley E, Chakwera S, Hussain-Alkhateeb L, et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: a systematic analysis. Lancet. 2023;402(10409):1261–71. - PubMed
-
- World Health Organization. Born too soon: Decade of action on preterm birth. Geneva; 2023.
-
- Ward RM, Beachy JC. Neonatal complications following preterm birth. BJOG: Int J Obstet Gynecol. 2003;110(Suppl 20):8–16. - PubMed
-
- Ferrero DM, Larson J, Jacobsson B, Di Renzo GC, Norman JE, et al. Cross-country individual participant analysis of 4.1 million singleton births in 5 countries with very high human development index confirms known associations but provides no biologic explanation for 2/3 of all preterm births. PLoS One. 2016;11(9):e0162506. - PMC - PubMed
-
- Goodfellow L, Care A, Alfirevic Z. Controversies in the prevention of spontaneous preterm birth in asymptomatic women: an evidence summary and expert opinion. BJOG: Int J Obstet Gynecol. 2021;128(2):177–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MS McLeod Research Fund Postdoctoral Fellowship/Women's and Children's Hospital Foundation
- Research Project Grant/Women's and Children's Hospital Foundation
- APP1135155/National Health and Medical Research Council
- APP1135155/National Health and Medical Research Council
- APP1135155/National Health and Medical Research Council
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous