Early-life serological profiles and the development of natural protective humoral immunity to Streptococcus pyogenes in a high-burden setting
- PMID: 40781379
- PMCID: PMC12532705
- DOI: 10.1038/s41591-025-03868-4
Early-life serological profiles and the development of natural protective humoral immunity to Streptococcus pyogenes in a high-burden setting
Erratum in
-
Publisher Correction: Early-life serological profiles and the development of natural protective humoral immunity to Streptococcus pyogenes in a high-burden setting.Nat Med. 2025 Oct;31(10):3570. doi: 10.1038/s41591-025-04017-7. Nat Med. 2025. PMID: 40993244 Free PMC article. No abstract available.
Abstract
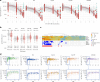
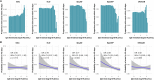
Streptococcus pyogenes leads to 500,000 deaths annually, many due to rheumatic heart disease in low-income settings. Limited understanding of natural protective immunity to S. pyogenes hinders vaccine development. Here we describe the evolution of serological profiles to conserved vaccine antigens and serotype-specific M proteins from birth and throughout the life course in The Gambia. As placentally transferred IgG waned after birth, serological evidence of new exposure was seen in 23% of infants during the first year of life. After culture-confirmed S. pyogenes events, the highest IgG increases occurred in children younger than 2 years of age after both pharyngeal and skin disease and asymptomatic carriage at both sites. Higher IgG levels against conserved vaccine antigens correlated with functional activity and were associated with protection from culture-confirmed events after adjustment for age and anti-M protein IgG levels. To our knowledge, our data provide the first evidence of protection associated with humoral immunity to conserved vaccine candidate antigens in humans.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: A.J.K. received training in immunoassay development and delivery from the GSK Vaccines Institute for Global Health, an affiliate of GlaxoSmithKline (GSK). GSK had no role in overall study design, data analysis or data interpretation for this study. O.R., M.C., E.B., L.R., L.M., M.I. and D.G.M. are employees of the GSK Vaccines Institute for Global Health. A.B. and P.R.S. are inventors on a submitted patent related to Streptococcus pyogenes vaccines. The other authors declare no competing interests.
Figures









References
-
- Carapetis, J. R., Steer, A. C., Mulholland, E. K. & Weber, M. The global burden of group A streptococcal diseases. Lancet Infect. Dis.5, 685–694 (2005). - PubMed
-
- Watkins, D. A. et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N. Engl. J. Med.377, 713–722 (2017). - PubMed
-
- WHO Preferred Product Characteristics for Group A Streptococcus Vaccines (World Health Organization, 2018); https://apps.who.int/iris/handle/10665/279142
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

