Impact of specific productivity and operation mode upon the biophysical properties of HIV-1 Gag-based virus-like particles
- PMID: 40781574
- PMCID: PMC12334472
- DOI: 10.1007/s00253-025-13560-9
Impact of specific productivity and operation mode upon the biophysical properties of HIV-1 Gag-based virus-like particles
Abstract
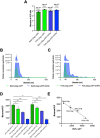
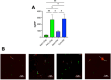
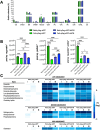
Virus-like particles (VLPs) are non-infective vaccine candidates that have gained interest given their natural ability to elicit strong immune responses. Particularly, HIV-1 Gag-based VLPs are one of the most described platforms for vaccine development, provided their ability for successful pseudotyping either by genetic engineering or click chemistry. When Gag polyprotein is recombinantly expressed, VLPs are naturally assembled in the vicinity of the cell membrane and then secreted by cell budding, taking part of the host cell membrane. Their properties are dependent upon the cell line and manufacturing method. Although great advancements toward the implementation of analytical methods have been made, VLP quality attributes are quite unclear whenever production is enhanced by metabolic engineering or process intensification strategies. This work offers a comparative study of VLP quality attributes upon transient gene expression (TGE) in HEK293 cell cultures operated in batch and perfusion mode. Moreover, the impact of specific productivity is also studied by ataxia telangiectasia mutated (ATM) gene silencing, which has been reported to enhance fourfold VLP production. A linear negative correlation was found between the ratio of Gag monomers/VLP and specific productivity. 3100 ± 100 monomers/VLP were obtained for the standard batch production, dropping to 1900 ± 100 and 800 ± 60 for the perfusion and batch ATM-knockdown conditions, respectively. Furthermore, functionalization rates were measured in terms of Cy5 per total particles (TP). Both perfusion-derived nanoparticles achieved functionalization rates of 2800 Cy5/TP. On the contrary, those nanoparticles produced in batch yielded functionalization rates below 1000 Cy5/TP. Moreover, a complete lipidome analysis revealed a relative decrease in the quantity of lipid/particle for all studied conditions in comparison to the standard batch production. Finally, all VLP samples were characterized to assess the impact of the differential physicochemical properties upon purification and stability rates. KEY POINTS: • VLP quality inversely correlates with Gag-specific productivity and operation mode. • Functionalization and lipid content drop with metabolic burden or ATM silencing. • Perfusion enables high VLP recovery and lyophilization with preserved morphology.
Keywords: ATM; Bioprocess development; Functionalization; HEK293; Lipidomics; Perfusion; TGE; Virus-like particles (VLPs).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: Not applicable. Conflict of interest: The authors declare no competing interests.
Figures


Similar articles
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
References
-
- Baldi L, Hacker DL, Adam M, Wurm FM (2007) Recombinant protein production by large-scale transient gene expression in mammalian cells: state of the art and future perspectives. Biotechnol Lett 29:677–684. 10.1007/s10529-006-9297-y - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

