Cytokine, Chemokine, and Neurofilament Light Chain Signatures in LGI1 Autoimmune Encephalitis
- PMID: 40781580
- PMCID: PMC12623847
- DOI: 10.1002/acn3.70158
Cytokine, Chemokine, and Neurofilament Light Chain Signatures in LGI1 Autoimmune Encephalitis
Abstract
Objectives: To investigate the value of cytokine, chemokine, and neurofilament light chain (NfL) concentrations in predicting relapse risk, chronic epilepsy, and functional impairment in LGI1 autoimmune encephalitis (AE).
Methods: Cytokines/chemokines (IL-1-beta, IL-2, IL-4, IL-5, IL-6, IL-8/CXCL8, IL-10, IL-12p70, IL-13, IL-17A, GM-CSF, TNF-alpha, IFN-gamma, CXCL9, CXCL10, CXCL13, BAFF) and NfL concentrations were measured in CSF and paired serum from LGI1-AE patients evaluated at Mayo Clinic (01/2015-02/2024), using a multiplex immunoassay system (ELLA, Bio-Techne) and correlated with clinical outcomes. A laboratory-based cohort of LGI1-IgG-positive patients and control cohorts, including patients with mixed non-inflammatory disorders (MNID), Alzheimer's disease (AD), and temporal lobe epilepsy (TLE) were analyzed.
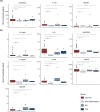
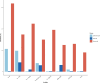
Results: Forty-four patients with LGI1-AE were included; 29 (66%) were male, with a median age of 68.5 years (range, 8-85). Median time from symptom onset to CSF sampling was eight months (IQR, 3-17); 19/42 (45%) experienced a clinical relapse and 27% developed chronic epilepsy. Serum IL-6, serum and CSF IL-8/CXCL8, and IL-17A were higher in LGI1-IgG positive patients than MNID (p < 0.05). TLE cytokine/chemokine profiles were similar to LGI1 AE; AD patients had lower serum IL-6 and CSF IL-8/CXCL8 (p = 0.04; p = 0.01), and higher serum IL-17A and GM-CSF (p = 0.004; p = 0.01) than LGI1-AE. Higher CSF IL-6 and IL-8/CXCL8 in LGI1-AE associated with clinical relapse (p < 0.05) and higher CSF NfL associated with chronic epilepsy (p = 0.01).
Conclusion: Elevations in IL-6, IL-8/CXCL8, and IL-17A were identified in this LGI1-AE cohort. CSF IL-6, IL-8/CXCL8, and NfL levels are potential prognostic biomarkers for risk of relapse and chronic epilepsy in LGI1-AE.
Keywords: Leucine‐rich glioma‐inactivated‐1; autoimmune seizures; biomarkers; epilepsy; faciobrachial dystonic seizures.
© 2025 The Author(s). Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
A.A., G.M., B.Y., V.K.P., J.W.B., E.P.F., G.S.D., C.L.H., A.S.L., J.R.M., Y.H., and M.T. report no relevant disclosures related to this manuscript. D.D. has consulted for UCB, Immunovant, Argenx, Arialys, and Astellas pharmaceuticals. All compensation for consulting activities is paid directly to Mayo Clinic. He is a named inventor on a filed patent that relates to KLHL11 as a marker of autoimmunity and germ cell tumor. He has patents pending for LUZP4‐IgG, cavin‐4‐IgG, and SKOR2‐IgG as markers of neurological autoimmunity. He has received funding from the DOD (CA210208 & PR220430), the David J. Tomassoni ALS Research Grant Program, and UCB. E.P.F. has served on advisory boards for Alexion, Genentech, Horizon Therapeutics, and UCB. He has received research support from UCB. He received royalties from UpToDate. Dr. Flanagan is a site principal investigator in a randomized clinical trial of Rozanolixizumab for relapsing myelin oligodendrocyte glycoprotein antibody‐associated disease run by UCB. Dr. Flanagan is a site principal investigator and a member of the steering committee for a clinical trial of satralizumab for relapsing myelin oligodendrocyte glycoprotein antibody‐associated disease run by Roche/Genentech. Dr. Flanagan is a Co‐Investigator on a study of ravulizumab for neuromyelitis optica spectrum disorder run by Alexion. Dr. Flanagan has given educational talks on neuromyelitis optica spectrum disorder funded by Alexion. Dr. Flanagan has received funding from the NIH (R01NS113828). Dr. Flanagan has received honoraria for editing and writing articles for The Continuum Lifelong Learning in Neurology Journal, which is a publication of the American Academy of Neurology. Dr. Flanagan is a member of the medical advisory board of the MOG project. Dr. Flanagan is an editorial board member of Neurology, Neuroimmunology and Neuroinflammation, The Journal of the Neurological Sciences, and Neuroimmunology Reports. A patent has been submitted on DACH1‐IgG as a biomarker of paraneoplastic autoimmunity. S.R.I. has received honoraria/research support from UCB, Immunovant, MedImmun, Roche, Janssen, Cerebral therapeutics, ADC therapeutics, BioHaven therapeutics, CSL Behring, and ONO Pharma and receives licensed royalties on patent application WO/2010/046716 entitled ‘Neurological Autoimmune Disorders’, and has filed two other patents entitled “Diagnostic method and therapy” (WO2019211633 and US app 17/051,930; PCT application WO202189788A1) and “Biomarkers” (WO202189788A1, US App 18/279,624; PCT/GB2022/050614). A.M. has patents issued for GFAP and MAP1B‐IgGs and patents pending for Septins‐5 and Septins‐7, and KLCHL11‐IgGs; and has consulted for Roche pharmaceuticals, without personal compensation. I.V. is a full‐time employee of F. Hoffmann‐La Roche Ltd. S.J.P. has received personal compensation for serving on scientific advisory boards or data safety monitoring boards for F. Hoffman‐LaRoche AG, Genentech, Arialys, and UCB. His institution has received compensation for serving as a consultant for Astellas, Alexion/AstraZeneca, and Viela Bio/MedImmune/Amgen. All compensation is paid to Mayo Clinic. He has received research support from Alexion/AstraZeneca, Viela Bio/MedImmune/Amgen, Roche/Genentech, and Adimmune. He has a patent, Patent# 8,889,102 (Application#12–678,350, Neuromyelitis Optica Autoantibodies as a Marker for Neoplasia)—issued; a patent, Patent# 9,891,219B2 (Application#12–573,942, Methods for Treating Neuromyelitis Optica (NMO) by Administration of Eculizumab to an individual that is Aquaporin‐4 (AQP4)‐IgG Autoantibody positive)‐issued and from which he has received royalties; and a patent for GFAP‐IgG, Septin‐5‐IgG, MAP1B‐IgG, Kelch‐like protein 11, and PDE10A pending. He is working as a consultant in the Mayo Clinic Neuroimmunology laboratory clinical service. The Mayo Clinic Neuroimmunology Laboratory commercially offers MOG‐IgG testing, but revenue accrued does not contribute to salary, research support, or personal income. A.Z. has patents submitted for DACH1‐IgG, PDE10A‐IgG, CMAKV‐IgG, Tenascin‐R‐IgG as biomarkers of neurological autoimmunity. She has received research funding from Roche/Genentech and the Center for MS and Autoimmune Neurology at Mayo Clinic relevant to this manuscript. She has consulted without personal compensation for Alexion Pharmaceuticals.
Figures


References
-
- Ariño H., Ruiz García R., Rioseras B., et al., “Frequency and Referral Patterns of Neural Antibody Studies During the COVID‐19 Pandemic: Experience From an Autoimmune Neurology Center,” Neurology Neuroimmunology & Neuroinflammation 10, no. 4 (2023): e200129, 10.1212/NXI.0000000000200129. - DOI - PMC - PubMed
-
- Irani S. R., Alexander S., Waters P., et al., “Antibodies to Kv1 Potassium Channel‐Complex Proteins Leucine‐Rich, Glioma Inactivated 1 Protein and Contactin‐Associated Protein‐2 in Limbic Encephalitis, Morvan's Syndrome and Acquired Neuromyotonia,” Brain 133, no. 9 (2010): 2734–2748, 10.1093/brain/awq213. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
