Elevated CD4⁺/CD8⁺ ratio and D-dimer as diagnostic biomarkers for Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis versus infectious mononucleosis in children: a retrospective case-control study
- PMID: 40781611
- PMCID: PMC12335061
- DOI: 10.1186/s12879-025-11440-1
Elevated CD4⁺/CD8⁺ ratio and D-dimer as diagnostic biomarkers for Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis versus infectious mononucleosis in children: a retrospective case-control study
Abstract
Objective: To identify potential diagnostic biomarkers distinguishing Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis (EBV-HLH) from infectious mononucleosis (EBV-IM) in pediatric patients using a retrospective case-control design.
Methods: This study enrolled a total of 160 pediatric patients, including 132 with Epstein-Barr virus-associated infectious mononucleosis (EBV-IM) and 28 with EBV-associated hemophagocytic lymphohistiocytosis (EBV-HLH). Serum levels of CD4⁺ T cells, CD8⁺ T cells, and D-dimer were quantified by flow cytometry and immunoturbidimetry, respectively. The CD4⁺/CD8⁺ ratio was calculated from absolute counts. These parameters, along with clinical and laboratory features, were compared between the EBV-IM and EBV-HLH groups. Binary logistic regression was used to analyze the risk factors for the progression of EBV infection to EBV-HLH. The clinical value of CD4⁺/CD8⁺ ratio and D-dimer levels in diagnosing EBV-HLH was assessed using receiver operating characteristic (ROC) curve analysis.
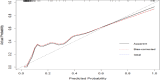
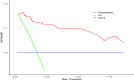
Results: The average age of the EBV-HLH group was significantly lower than that of the IM group (p < 0.05). The EBV-HLH group had significantly higher levels of NLR(Neutrophil to Lymphocyte Ratio), PLR(Platelet to Lymphocyte Ratio), D-dimer, EBV-DNA, disease duration, CD4⁺/CD8⁺ ratio, and AST/ALT compared to the IM group (p < 0.05).Binary logistic regression analysis indicated that a higher CD4⁺/CD8⁺ ratio (OR = 17.60, 95% CI: 1.89-163.64; p < 0.05) and elevated D-dimer levels (OR = 1.31 per 1 mg/L increase, 95% CI: 1.08-1.59; p < 0.05) were significantly associated with EBV-HLH. Building upon the identified associations, we evaluated the diagnostic performance of these biomarkers. ROC curve analysis demonstrated that CD4⁺/CD8⁺ ratio > 0.455 (Youden index = 0.638, sensitivity = 92.4%, specificity = 71.4%) and D-dimer > 1.675 mg/L (Youden index = 0.683, sensitivity = 82.6%, specificity = 85.7%) optimally discriminated EBV-HLH from EBV-IM. The combined model significantly enhanced diagnostic accuracy (Youden index = 0.811), with AUC values of 0.837 (95%CI: 0.76-0.91), 0.869 (95%CI: 0.80-0.94), and 0.962 (95%CI: 0.935-0.989) for CD4⁺/CD8⁺ ratio, D-dimer, and their combination, respectively.
Conclusion: Elevated CD4⁺/CD8⁺ ratio and D-dimer serve as potential diagnostic biomarkers for pediatric EBV-HLH. Their combined detection enhances differentiation from EBV-IM, though validation through prospective studies is warranted.
Keywords: CD4⁺/CD8⁺ ratio; D-dimer; Epstein-Barr virus infections; Hemophagocytic; Infectious mononucleosis; Lymphohistiocytosis; Pediatrics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Ethics Committee of Anhui Provincial Children’s Hospital (Ethical Approval No.: EYLL-2023-041) and conducted in accordance with the Declaration of Helsinki. Patient data were de-identified prior to analysis and accessible only to the research team. The Ethics Committee formally waived informed consent under national regulations (Article 39, Regulations on Ethical Review of Biomedical Research Involving Humans, China 2016) due to the retrospective design. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
High Epstein-Barr Virus DNA Load in T Cells Predicts Hemophagocytic Lymphohistiocytosis.J Infect Dis. 2025 Jul 30;232(1):212-219. doi: 10.1093/infdis/jiaf065. J Infect Dis. 2025. PMID: 40036428
-
The ability of CD4dimCD8+ T cells to distinguish between Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis and pediatric infectious mononucleosis.Immunol Res. 2025 Jan 30;73(1):43. doi: 10.1007/s12026-025-09597-7. Immunol Res. 2025. PMID: 39883282
-
Clinical and prognostic significance of whole blood Epstein-Barr virus DNA in patients with primary hemophagocytic lymphohistiocytosis: a retrospective observational study from China.Ann Hematol. 2025 Jul;104(7):3597-3607. doi: 10.1007/s00277-025-06447-2. Epub 2025 Jun 19. Ann Hematol. 2025. PMID: 40536720 Free PMC article.
-
Assessment of immunochemotherapy and stem cell transplantation on EBV-associated hemophagocytic lymphohistiocytosis in children: a systematic review and meta analysis.Eur Rev Med Pharmacol Sci. 2012 May;16(5):672-8. Eur Rev Med Pharmacol Sci. 2012. PMID: 22774410
-
Antiviral agents for infectious mononucleosis (glandular fever).Cochrane Database Syst Rev. 2016 Dec 8;12(12):CD011487. doi: 10.1002/14651858.CD011487.pub2. Cochrane Database Syst Rev. 2016. PMID: 27933614 Free PMC article.
References
-
- Paul RL, AnnaCarin H, Melissa H, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465–77. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

