The effect of Polyvinylpyrrolidone-Sodium hyaluronate gel on palatal wound healing: a randomized controlled clinical trial
- PMID: 40781616
- PMCID: PMC12333267
- DOI: 10.1186/s12903-025-06677-w
The effect of Polyvinylpyrrolidone-Sodium hyaluronate gel on palatal wound healing: a randomized controlled clinical trial
Abstract
Background: This study evaluates the effect of polyvinylpyrrolidone sodium hyaluronate gel (Gelclair®) on palatal wound healing, pain, and bleeding following free gingival graft surgery (FGG).
Methods: Thirty-two patients undergoing FGG were randomly assigned to two groups: the test group received Gelclair® and chlorhexidine mouthwash, while the control group received only chlorhexidine. Patients were called at first, third, seventh, fourteenth and twenty-eighth postoperative days and, Visual Analog Scale (VAS), Wound Healing Index (WHI), hydrogen peroxide (H₂O₂) bubbling scores and bleeding situations were recorded. The Friedman test was used for repeated measures, with the Wilcoxon signed-rank test as post-hoc, and the Mann-Whitney U test for between-group comparisons.
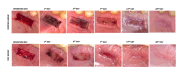
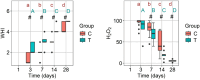
Results: H₂O₂ test values significantly decreased and WHI values significantly increased over time in both groups (p < 0.050). Test and control groups comparison at time points showed the test group showed significantly lower H₂O₂ test values on days 7, 14, and 28 (p < 0.050) and higher WHI values on days 3, 7, 14, and 28 compared to control group. VAS pain, chewing, and burning scores were also significantly lower in the test group on days 1, 3, 7, and 14 compared to control. While on day 1, the control group had a bleeding rate of 105 times higher than the test group (p < 0.001), no significant differences were observed on days 3 and 7.
Conclusions: The findings suggest that Gelclair® promotes wound healing and reduces discomfort and bleeding in the palatal donor area after FGG.
Trial registration number: NCT06610331; Retrospectively registered on 23/09/2024.
Keywords: Donor site; Free gingival graft; Hyaluronic acid; Pain; Postoperative complications; Wound healing.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Ethics Committee of Bolu Abant Izzet Baysal University (22/08/2023/255) and conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013. Also, this study was registered on ClinicalTrials.gov (NCT06610331). All participants have signed informed consent forms and given their permission to use clinical information/video/photographic material for the analysis and publication of this study. Consent for publication: All participants have signed informed consent forms and given their permission to use clinical information/video/photographic material for the analysis and publication of this study. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Patient-reported outcome measures comparing povidone iodine rinse and chlorhexidine rinse for dental implant therapy: a randomized controlled trial.BMC Oral Health. 2025 Jul 23;25(1):1233. doi: 10.1186/s12903-025-06560-8. BMC Oral Health. 2025. PMID: 40702480 Free PMC article. Clinical Trial.
-
Palatal pre-suturing effects on hemostasis and morbidity in connective tissue grafting: A randomized controlled trial.J Periodontol. 2025 Aug 17. doi: 10.1002/jper.11392. Online ahead of print. J Periodontol. 2025. PMID: 40820445
-
Clinical effect of CGF blood concentration factor in extracting supernumerary teeth in the middle and high positions of the upper palate.SLAS Technol. 2025 Aug;33:100321. doi: 10.1016/j.slast.2025.100321. Epub 2025 Jun 19. SLAS Technol. 2025. PMID: 40543714
-
Antibiotics and antiseptics for venous leg ulcers.Cochrane Database Syst Rev. 2013 Dec 23;(12):CD003557. doi: 10.1002/14651858.CD003557.pub4. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2014 Jan 10;(1):CD003557. doi: 10.1002/14651858.CD003557.pub5. PMID: 24363048 Updated.
-
Antiseptics for burns.Cochrane Database Syst Rev. 2017 Jul 12;7(7):CD011821. doi: 10.1002/14651858.CD011821.pub2. Cochrane Database Syst Rev. 2017. PMID: 28700086 Free PMC article.
References
-
- Tavelli L, Barootchi S, Ravidà A, Oh T-J, Wang H-L. What is the safety zone for palatal soft tissue graft harvesting based on the locations of the greater palatine artery and foramen?? A systematic review. J Oral Maxillofac Surg. 2019;77:e2711–9. - PubMed
-
- Chambrone L, Tatakis DN. Periodontal soft tissue root coverage procedures: a systematic review from the AAP regeneration workshop. J Periodontol. 2015;86:S8–51. - PubMed
-
- Wang RE, Lang NP. Ridge preservation after tooth extraction. Clin Oral Implants Res. 2012;23:147–56. - PubMed
-
- Thoma DS, Naenni N, Figuero E, Hämmerle CHF, Schwarz F, Jung RE, et al. Effects of soft tissue augmentation procedures on peri-implant health or disease: a systematic review and meta-analysis. Clin Oral Implants Res. 2018;29:32–49. - PubMed
-
- Sanz M, Simion M. Surgical techniques on periodontal plastic surgery and soft tissue regeneration: consensus report of group 3 of the 10th European workshop on periodontology. J Clin Periodontol. 2014;41(Suppl 15):S92–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

