Fibrous dysplasia/McCune-Albright syndrome: state-of-the-art advances, pathogenesis, and basic/translational research
- PMID: 40781626
- PMCID: PMC12335126
- DOI: 10.1186/s13023-025-03909-8
Fibrous dysplasia/McCune-Albright syndrome: state-of-the-art advances, pathogenesis, and basic/translational research
Abstract
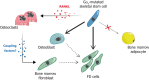
Fibrous dysplasia/McCune Albright syndrome (FD/MAS) is a rare genetic disease caused by postzygotic activating variants in the GNAS gene, encoding the α subunit of stimulatory G protein (Gαs). Although multiple organs may be involved, skeletal lesions usually represent the most severe and least treatable expression of the disease, leading to bone deformities, spontaneous fractures, and chronic pain that severely reduce patients' quality of life.The recognition of the causative Gαs variants and the consequent ligand-independent activation of the adenylyl cyclase/cAMP/PKA pathway has provided a clear molecular explanation to most extra-skeletal pathologies of FD/MAS, leading to the development of effective therapeutic approaches. In contrast, a detailed understanding of the cellular and molecular mechanisms that act downstream of the Gαs pathway to generate FD bone lesions and clinical expression thereof remain elusive. Multiple key issues remain to be addressed, including some questions that have recently emerged such as the interaction between mutated and non-mutated cells and the role of the latter in the development of the fibrotic tissue.In this review, we provide a summary of the proof-of-concept, preclinical data, and experimental tools that have emerged to date from basic and translational studies on FD and represent the background for future research on the pathogenesis and treatment of this rare disease.
Keywords: Bone remodeling; GNAS; Mouse models; Osteoclastogenesis; Osteogenesis; RANKL; Rare disease; Skeletal stem cell.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: JFC has received grant support from Novartis and honoraria from Abbvie, Alexion, Ultragenyx, and Kyowa Kirin. ECH serves in an unpaid capacity on the Medical Registry Advisory Board of the International Fibrodysplasia Ossificans Progressiva Association (FOP); the Fibrous Dysplasia Foundation Medical Advisory Board and Scientific Advisory Board; and the International Clinical Council on FOP. ECH receives clinical trials support through his institution from Clementia Pharmaceuticals, an Ipsen Company; Ipsen Pharmaceuticals; Ascendis Bio, and ashibio.
Figures


References
-
- Bianco P, Riminucci M, Majolagbe A, Kuznetsov SA, Collins MT, Mankani MH, et al. Mutations of the GNAS1 gene, stromal cell dysfunction, and osteomalacic changes in non-McCune-Albright fibrous dysplasia of bone. J Bone Min Res. 2000;15:120–8. - PubMed
-
- Idowu BD, Al-Adnani M, O’Donnell P, Yu L, Odell E, Diss T, et al. A sensitive mutation‐specific screening technique for GNAS1 mutations in cases of fibrous dysplasia: the first report of a codon 227 mutation in bone. Histopathology. 2007;50:691–704. - PubMed
-
- Shenker A, Weinstein LS, Sweet DE, Spiegel AM. An activating Gs alpha mutation is present in fibrous dysplasia of bone in the McCune-Albright syndrome. J Clin Endocrinol Metab. 1994;79:750–5. - PubMed
-
- Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune–Albright syndrome. N Engl J Med. 1991;325:1688–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

