Lonicerae flos and turmeric extracts alleviate necrotic enteritis in broilers by modulating gut-liver health and microbiota
- PMID: 40781642
- PMCID: PMC12333254
- DOI: 10.1186/s40104-025-01246-1
Lonicerae flos and turmeric extracts alleviate necrotic enteritis in broilers by modulating gut-liver health and microbiota
Abstract
Background: Necrotic enteritis (NE) can cause intestinal barrier dysfunction in broilers, leading to secondary liver injury (SLI). In this process, the gut-liver axis plays a crucial role. Lonicerae flos and turmeric extracts (LTE), containing chlorogenic acid and curcumin, have been reported to possess anti-inflammatory, and antioxidant properties. Based on these potential biological benefits, this study aims to investigate the reparative effects of LTE on the intestinal barrier dysfunction in NE-infected broilers and assess its therapeutic efficacy in alleviating SLI. By elucidating the regulatory mechanisms of LTE on gut-liver axis health, this research provides new insights into the prevention and treatment of NE in broilers.
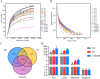
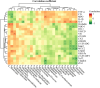
Results: LTE improved body weight and average daily gain while reducing intestinal lesion scores, coccidia oocysts, and Clostridium perfringens counts in NE broilers (P < 0.05). LTE enhanced intestinal morphology and up-regulated the expression of tight junction protein genes (CLDN1, TJP1) and MUC2, suppressed pro-inflammatory cytokine and myeloperoxidase (MPO) levels, and minimized endotoxin (ET) accumulation in NE broilers (P < 0.05). Furthermore, LTE alleviated oxidative stress in ileal cells and protected mitochondrial structure and function in NE broilers. NE infection induced intestinal permeability in broilers, leading to increased serum pro-inflammatory cytokines and intestinal-derived endotoxin levels, which caused liver damage. LTE significantly reduced liver pathologic damage, pro-inflammatory cytokine levels, aspartate transaminase, alanine aminotransferase, and ROS levels in NE broilers (P < 0.05). Additionally, 16S rRNA sequencing revealed that NE significantly increased the relative abundance of Barnesiella and decreased the relative abundance of Bacteroidota, Desulfobacterota and Bacteroides in the cecum of broilers. LTE enhanced intestinal microbiota diversity and reduced the segregation of intestinal microbiota induced by NE infection.
Conclusions: In summary, LTE can alleviate NE and SLI by modulating the microbiota, inhibiting inflammation and oxidative stress, and ameliorating mitochondrial dysfunction, thereby enhancing gut-liver axis health and growth performance.
Keywords: Chlorogenic acid; Curcumin, Microbiota; Mitochondrial dysfunction; Necrotic enteritis; Secondary liver injury.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Animal Ethics Committee of China Agricultural University (AW31903202-1-1) and was conducted in accordance with the Guidelines for Laboratory Animals established by the Chinese Ministry of Science and Technology (Beijing, China). Consent for publication: Not applicable. Competing interests: One of the co-authors is an employee of the company that provides the LTE product. The authors have no other financial interests related to this work, and they affirm that the research was conducted with full scientific independence and without external influence. The authors declare that they have no competing interests.
Figures






Similar articles
-
Dietary curcumin supplementation enhances growth performance and anti-inflammatory functions by modulating gut microbiota, microbiota-derived metabolites, and expression of inflammation-related genes in broilers.J Anim Sci. 2024 Jan 3;102:skae296. doi: 10.1093/jas/skae296. J Anim Sci. 2024. PMID: 39324614
-
Phloretin supplementation ameliorates intestinal injury of broilers with necrotic enteritis by alleviating inflammation, enhancing antioxidant capacity, regulating intestinal microbiota, and producing plant secondary metabolites.Poult Sci. 2025 Jul;104(7):105187. doi: 10.1016/j.psj.2025.105187. Epub 2025 Apr 29. Poult Sci. 2025. PMID: 40367711 Free PMC article.
-
Effect of coated organic acids on growth performance, Clostridium perfringens colonization, gut integrity and immune response in broilers challenged with subclinical necrotic enteritis.Poult Sci. 2025 Jun 28;104(10):105504. doi: 10.1016/j.psj.2025.105504. Online ahead of print. Poult Sci. 2025. PMID: 40628145 Free PMC article.
-
Efficacy of Bacillus subtilis probiotic in preventing necrotic enteritis in broilers: a systematic review and meta-analysis.Avian Pathol. 2024 Dec;53(6):451-466. doi: 10.1080/03079457.2024.2359596. Epub 2024 Jul 3. Avian Pathol. 2024. PMID: 38776185
-
The Gordon Memorial Lecture: Steering the gut microbiome for improved health and welfare in broilers.Br Poult Sci. 2025 Aug;66(4):419-428. doi: 10.1080/00071668.2025.2488014. Epub 2025 Jun 6. Br Poult Sci. 2025. PMID: 40476348 Review.
References
-
- Gu C, Lillehoj HS, Sun Z, Lee Y, Zhao H, Xianyu Z, et al. Characterization of virulent netB+/tpeL+Clostridium perfringens strains from necrotic enteritis-affected broiler chicken farms. Avian Dis. 2019;63(3):461–7. 10.1637/11973-092018-Reg.1. - PubMed
-
- Moore RJ. Necrotic enteritis predisposing factors in broiler chickens. Avian Pathol. 2016;45(3):275–81. 10.1080/03079457.2016.1150587. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

