Serological assessment of PRO-C16 (type XVI collagen formation) reflects intestinal fibrostenotic strictures in patients with crohn's disease
- PMID: 40781657
- PMCID: PMC12333124
- DOI: 10.1186/s12876-025-04146-w
Serological assessment of PRO-C16 (type XVI collagen formation) reflects intestinal fibrostenotic strictures in patients with crohn's disease
Abstract
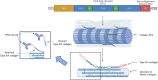
Background: Fibrostenotic stricturing disease affects 30-50% of patients with Crohn's disease (CD), leading to intestinal resection. Currently, there exists a great medical need to identify biomarkers related to fibrostenotic strictures for optimized patient management. Thus, we investigated PRO-C16 as a biomarker for intestinal fibrosis in patients with CD.
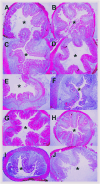
Methods: Human serum from two independent cohorts of CD patients (cohort 1: n = 44, cohort 2: n = 52), healthy subjects (n = 37), and serum from a chronic rat dextran sodium sulfate (DSS) colitis model were included. The Montreal classification for CD disease behavior was applied for patient phenotyping.
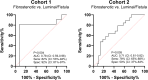
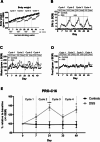
Results: PRO-C16 was elevated in patients with CD compared to healthy donors (P < 0.001), and in CD patients with fibrostenotic strictures in both cohorts. Furthermore, PRO-C16 was able to separate CD patients with strictures(B2) from CD patients without strictures (B1 and B3) (Cohort 1 [P < 0.01, AUC:0.75], and Cohort 2 [P < 0.05, AUC:0.71). In the chronic DSS rat colitis model, PRO-C16 was elevated after the second and fourth cycles of DSS, reflecting collagen deposition in that model.
Conclusion: The biomarker PRO-C16 was associated with a stricturing disease phenotype, indicating that PRO-C16 may be a potential marker of intestinal fibrosis in CD, with the potential to aid in the clinical development of novel stromal-immune therapeutic agents.
Keywords: Biomarkers; COL16; Collagen; Crohn's disease; Intestinal fibrosis; Stenosis; Type XVI collagen.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All patients filed informed consent, and the study was approved by the local ethical committee (Ethics committee of the Fondazione IRCCS, protocol number 20100039131, approval no. E_20100039131). The DSS in vivo study’s ethical guidelines were followed in accordance with the legislation and under the ethical approval of the “Dyreforsøgstilsynet” (agreement number: 2017-15-0201-01171), and male Sprague Dawley rats were purchased from Envigo+++. This study adheres to the Declaration of Helsinki for ethics approval and patient consent to participate. Consent for publication: Not applicable. Competing interests: J.H. Mortensen, L. Langholm, T. Manon-Jensen, A-C. Bay-Jensen, and M.A. Karsdal are employed at Nordic Bioscience A/S which is a company involved in the discovery and development of biochemical biomarkers. T. Manon-Jensen, A-C. Bay-Jensen, and M.A. Karsdal own stocks in Nordic Bioscience. D. Ruane is employed at Janssen Immunology which is a company involved in drug development.
Figures


References
-
- Bos S, Laukens D. Metabolic modulation during intestinal fibrosis. J Dig Dis. 2020;21:319–25. - PubMed
-
- Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimising response to anti-TNF therapy in crohn’s disease - algorithm for practical management. Aliment Pharmacol Ther. 2016;43:30–51. - PubMed
-
- Ratzinger S, Eble JA, Pasoldt A, Opolka A, Rogler G, Grifka J, et al. Collagen XVI induces formation of focal contacts on intestinal myofibroblasts isolated from the normal and inflamed intestinal tract. Matrix Biol. 2010;29:177–93. - PubMed

