Baseline values for Quantra® QPlus® in healthy pregnant women at term and comparison to standard laboratory coagulation values: a prospective observational study
- PMID: 40781663
- PMCID: PMC12333236
- DOI: 10.1186/s12884-025-07927-z
Baseline values for Quantra® QPlus® in healthy pregnant women at term and comparison to standard laboratory coagulation values: a prospective observational study
Abstract
Background: Hypofibrinogenemia is associated with progression from moderate to severe postpartum hemorrhage (PPH). Early recognition and replacement of fibrinogen are emphasized during the management of PPH. The Quantra® QPlus® System, a novel point-of-care viscoelastic testing (POCVT) device, has been designed to provide rapid assessment of hemostasis. We aimed to evaluate the correlation between Quantra parameters and standard laboratory coagulation tests, and to establish baseline Quantra values in healthy term pregnant women.
Methods: Healthy pregnant women in labor or scheduled for elective cesarean delivery (CD) were enrolled in our prospective observational study. Blood samples for Quantra and standard laboratory coagulation tests were taken simultaneously. Quantra values, standard laboratory coagulation test, time of blood collected, and time to the result were recorded. We compared the baseline values between CD and labor group using a t-test, and the correlation between Quantra and standard laboratory coagulation test was calculated using partial Pearson correlation.
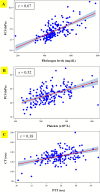
Results: 170 healthy pregnant women were included; 126 cases were in the CD group, and 44 patients were in the labor group. We found a strong correlation between Quantra Fibrinogen contribution to Clot Stiffness (FCS) and fibrinogen level (r = 0.67). The median [interquartile range] time of fibrinogen results by Quantra was 36 [28, 48] minutes faster than the standard laboratory coagulation tests. Baseline ranges for Quantra values, which were not significantly different between the two groups, demonstrated hyperfibrinogenemia during pregnancy.
Conclusion: Quantra is a novel POCVT device that rapidly provides coagulation status in pregnant women. The strong correlation between FCS and fibrinogen level can be helpful for early recognition of hypofibrinogenemia for the management of PPH.
Keywords: Blood coagulation test; Hypofibrinogenemia; Point-of-care viscoelastic testing; Postpartum hemorrhage.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Yale University (IRB no.2000031959). Informed consent was obtained from all individual participants included in the study. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- O’Brien KL, Shainker SA, Lockhart EL. Transfusion management of obstetric hemorrhage. Transfus Med Rev. 2018;32(4):249–. 10.1016/j.tmrv.2018.05.003. 55. - PubMed
-
- Say L, Chou D, Gemmill A, Tunçalp Ö, Moller A-B, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Global Health. 2014;2(6):e323–33. 10.1016/S2214-109X(14)70227-X. - PubMed
-
- Anderson JM, Etches D. Prevention and management of postpartum hemorrhage. Am Fam Physician. 2007;75(6):875–82. - PubMed
-
- Naz H, Sarwar I, Fawad A, Nisa AU. Maternal morbidity and mortality due to primary PPH–experience at Ayub teaching hospital Abbottabad. J Ayub Med Coll Abbottabad. 2008;20(2):59–65. - PubMed
-
- Collins PW, Bell SF, de Lloyd L, Collis RE. Management of postpartum haemorrhage: from research into practice, a narrative review of the literature and the Cardiff experience. Int J Obstet Anesth. 2019;37:106–17. 10.1016/j.ijoa.2018.08.008. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

