Cellular senescence in cancer: from mechanism paradoxes to precision therapeutics
- PMID: 40781676
- PMCID: PMC12333312
- DOI: 10.1186/s12943-025-02419-2
Cellular senescence in cancer: from mechanism paradoxes to precision therapeutics
Abstract
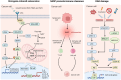
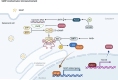
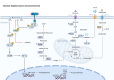
Cellular senescence is a double-edged sword in cancer biology, functioning as both a tumor-suppressive mechanism and a driver of malignancy. Initially, senescence acts as a protective barrier by arresting the proliferation of damaged or oncogene-expressing cells via pathways such as oncogene-induced senescence and the DNA damage response. However, persistent senescence-associated secretory phenotype and metabolic reprogramming in senescent cells create a pro-inflammatory, immunosuppressive tumor microenvironment, fueling cancer progression, therapy resistance, and metastasis. This comprehensive review systematically examines the molecular mechanisms of senescence across diverse cancers, spanning digestive, reproductive, urinary, respiratory, nervous, hematologic, endocrine, and integumentary systems, and elucidates its context-dependent roles in tumor suppression and promotion. We highlight groundbreaking therapeutic innovations, including precision senolytics, senomorphics, and combinatorial strategies integrating immunotherapy, metabolic interventions, and epigenetic modulators. The review also addresses microenvironment remodeling and cutting-edge technologies for dissecting senescence heterogeneity, epigenetic clocks for biological age prediction, and microbiome engineering to modulate senescence. Despite their promise, challenges such as off-target effects, biomarker limitations, and cellular heterogeneity underscore the need for precision medicine approaches. Finally, we propose future directions to harness senescence as a dynamic therapeutic target, offering transformative potential for cancer treatment.
Keywords: Cancer; Senescence; Therapeutic innovation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: All authors consent to publication. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Targeting Senescence: A Review of Senolytics and Senomorphics in Anti-Aging Interventions.Biomolecules. 2025 Jun 13;15(6):860. doi: 10.3390/biom15060860. Biomolecules. 2025. PMID: 40563501 Free PMC article. Review.
-
Interplay between tumor mutation burden and the tumor microenvironment predicts the prognosis of pan-cancer anti-PD-1/PD-L1 therapy.Front Immunol. 2025 Jul 24;16:1557461. doi: 10.3389/fimmu.2025.1557461. eCollection 2025. Front Immunol. 2025. PMID: 40777041 Free PMC article.
-
Persistent accumulation of therapy-induced senescent cells: an obstacle to long-term cancer treatment efficacy.Int J Oral Sci. 2025 Aug 1;17(1):59. doi: 10.1038/s41368-025-00380-w. Int J Oral Sci. 2025. PMID: 40750580 Free PMC article. Review.
-
Senescence in cancer.Cancer Cell. 2025 Jul 14;43(7):1204-1226. doi: 10.1016/j.ccell.2025.05.015. Epub 2025 Jun 12. Cancer Cell. 2025. PMID: 40513577 Review.
-
ATM and p53 in aging and cancer: a double-edged sword in genomic integrity.Biogerontology. 2025 May 5;26(3):102. doi: 10.1007/s10522-025-10249-4. Biogerontology. 2025. PMID: 40323544 Review.
References
-
- Lopez-Otin C, et al. Meta-hallmarks of aging and cancer. Cell Metab. 2023;35(1):12–35. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

