Mathematical modeling of dengue virus transmission: exploring vector, vertical, and sexual pathways with sensitivity and bifurcation analysis
- PMID: 40781679
- PMCID: PMC12333153
- DOI: 10.1186/s12879-025-11435-y
Mathematical modeling of dengue virus transmission: exploring vector, vertical, and sexual pathways with sensitivity and bifurcation analysis
Abstract
Background: Dengue virus (DENV) remains a critical global health threat, particularly in tropical and subtropical regions. Traditional models primarily focus on mosquito-borne transmission, overlooking alternative pathways such as vertical and sexual transmission. This study develops a comprehensive mathematical model that integrates multiple transmission routes to improve understanding of dengue dynamics and inform effective control strategies.
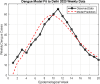
Methods: We develop a compartmental SEIR-based model that captures dengue virus transmission through mosquito vectors, vertical (transovarial), and sexual routes. The model undergoes rigorous mathematical analysis to derive equilibrium points and assess their stability. Both local and global sensitivity analyses are performed to identify key drivers of disease dynamics. Additionally, the model is calibrated using weekly dengue incidence data from Delhi, India, to validate its predictive capacity.
Results:
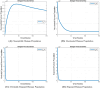
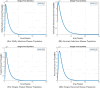
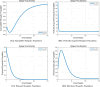
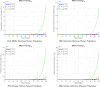
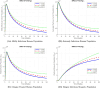
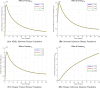
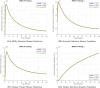
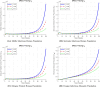
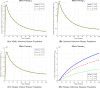
The sensitivity analysis identifies the most influential parameters driving transmission. Although the human-to-human contact rate (sexual transmission) has a high sensitivity index, the actual contribution of sexual transmission to the basic reproduction number () is biologically negligible—approximately 0.01704 out of a total
of 0.02, i.e., less than 1%. In contrast, mosquito-borne transmission remains the dominant route. The vaccination rate exhibits a negative sensitivity index, indicating its suppressive impact on disease spread. Numerical simulations reveal that dengue can persist even when
, indicating backward bifurcation, which necessitates enhanced intervention strategies beyond just reducing
.
Conclusion:
The model reveals that dengue can persist even when , due to backward bifurcation. Although sexual transmission contributes less than 1% to
under current estimates, vector control and vaccination remain the most critical strategies. Incorporating climate and mobility dynamics in future studies can further enhance model accuracy and policy relevance.
Keywords: Dengue intervention strategies; Epidemiological modeling; Human-to-human transmission; Mosquito-to-mosquito transmission; Vector-borne infections.
Conflict of interest statement
Declarations. Clinical trial number: Not applicable. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Significance of vertical transmission of arboviruses in mosquito-borne disease epidemiology.Parasit Vectors. 2025 Apr 9;18(1):137. doi: 10.1186/s13071-025-06761-8. Parasit Vectors. 2025. PMID: 40205559 Free PMC article.
-
Mathematical models for dengue fever epidemiology: A 10-year systematic review.Phys Life Rev. 2022 Mar;40:65-92. doi: 10.1016/j.plrev.2022.02.001. Epub 2022 Feb 15. Phys Life Rev. 2022. PMID: 35219611 Free PMC article.
-
Dynamics of vector competence for dengue virus type 2 in rural and urban populations of Aedes albopictus: implications for infectious disease control.Parasit Vectors. 2025 Jun 1;18(1):201. doi: 10.1186/s13071-025-06826-8. Parasit Vectors. 2025. PMID: 40452062 Free PMC article.
-
Natural vertical transmission of dengue virus in Aedes aegypti and Aedes albopictus: a systematic review.Parasit Vectors. 2018 Feb 1;11(1):77. doi: 10.1186/s13071-018-2643-9. Parasit Vectors. 2018. PMID: 29391071 Free PMC article.
-
Role of vector phenotypic plasticity in disease transmission as illustrated by the spread of dengue virus by Aedes albopictus.Nat Commun. 2024 Sep 7;15(1):7823. doi: 10.1038/s41467-024-52144-5. Nat Commun. 2024. PMID: 39242617 Free PMC article.
References
-
- Silva MMO, Tauro LB, Kikuti M, Anjos RO, Santos VC, Gonçalves TSF, Paploski IAD, et al. Concomitant transmission of dengue, chikungunya, and Zika viruses in Brazil: Clinical and epidemiological findings from surveillance for acute febrile illness. Clin Infect Dis. 2019;69(8):1353–9. 10.1093/cid/ciy1083. - PMC - PubMed
-
- Pandey HR, Phaijoo GR. Analysis of dengue infection transmission dynamics in Nepal using fractional order mathematical modeling. Chaos, Solitons Fractals X. 2023;11:100098.
-
- Thenmozhi V, Hiriyan JG, Tewari SC, Samuel PP, Paramasivan R, Rajendran R, et al. Natural vertical transmission of dengue virus in Aedes albopictus (Diptera: Culicidae) in Kerala, a Southern Indian State. Jpn J Infect Dis. 2007;60(5):245–9. 10.7883/yoken.jjid.2007.245. - PubMed
LinkOut - more resources
Full Text Sources

