Herbal products use during pregnancy and postpartum: study of consumption and user profile in Catalonia
- PMID: 40781711
- PMCID: PMC12335089
- DOI: 10.1186/s12906-025-05008-4
Herbal products use during pregnancy and postpartum: study of consumption and user profile in Catalonia
Abstract
Background: The prevalence of herbal products (HPs) consumption among pregnant and postpartum women, the factors driving their use or the main sources of recommendation have never been studied in Spain or Catalonia. Investigating its prevalence of use during critical phases of development is crucial for providing guidance to health professionals.
Methods: A validated questionnaire, containing general data on socio-demographic status, lifestyle, maternal health data and its association with HP consumption, was performed in online personal interviews among women living in Catalonia between pregnancy week 22 and postpartum month 9.
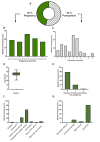
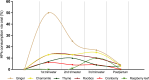
Results: We identified a higher percentage of HPs consumption compared to other European countries, while the 5 most consumed products were similar to the products described to be consumed by pregnant women in other countries. The most frequently consumed HPs were ginger (28%), chamomile (9%), thyme (7%), rooibos (6%), cranberry (4%), and raspberry leaf (4%), and we identified specific temporal patterns of consumption for several of them, depending on the trimester of pregnancy. Furthermore, we found a significant relationship between women consuming oral HPs and the opinion that "pregnant women should preferably consume herbal remedies rather than conventional medicines".
Conclusions: We provide evidence that women consuming HPs during pregnancy are not defined by a specific profile and therefore, healthcare professionals should be aware that any woman could potentially consume HPs during this period.
Keywords: Consumption; Herbal medicine; Interviews; Prenatal and postpartum health; Traditional use.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Given that the research plan involved collecting information from human subjects through personal interviews, approval was obtained from the Bioethics Committee of the University of Barcelona (approved by the Institutional Review Board, code: IRB00003099) on March 30th, 2020. Informed consent was requested from each participant, ensuring their freedom to decide whether or not to take part in the study, as well as to withdraw at any point if they so wished. To protect the privacy of participants and ensure data confidentiality, no names or personally identifiable information were disclosed. The study was conducted in accordance with the ethical principles set forth in the Declaration of Helsinki. Consent for publication: Not applicable. Clinical trial number: Not applicable. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Thromboprophylaxis during pregnancy and the puerperium: a systematic review and economic evaluation to estimate the value of future research.Health Technol Assess. 2024 Mar;28(9):1-176. doi: 10.3310/DFWT3873. Health Technol Assess. 2024. PMID: 38476084 Free PMC article.
-
Incentives for increasing prenatal care use by women in order to improve maternal and neonatal outcomes.Cochrane Database Syst Rev. 2015 Dec 15;2015(12):CD009916. doi: 10.1002/14651858.CD009916.pub2. Cochrane Database Syst Rev. 2015. PMID: 26671418 Free PMC article.
-
Prophylactic interventions after delivery of placenta for reducing bleeding during the postnatal period.Cochrane Database Syst Rev. 2013 Nov 26;2013(11):CD009328. doi: 10.1002/14651858.CD009328.pub2. Cochrane Database Syst Rev. 2013. PMID: 24277681 Free PMC article.
-
Preexisting Diabetes and Pregnancy: An Endocrine Society and European Society of Endocrinology Joint Clinical Practice Guideline.Eur J Endocrinol. 2025 Jun 30;193(1):G1-G48. doi: 10.1093/ejendo/lvaf116. Eur J Endocrinol. 2025. PMID: 40652450
-
Antibiotic regimens for management of intra-amniotic infection.Cochrane Database Syst Rev. 2014 Dec 19;2014(12):CD010976. doi: 10.1002/14651858.CD010976.pub2. Cochrane Database Syst Rev. 2014. PMID: 25526426 Free PMC article.
References
-
- World Health Organization (WHO). Guidelines on safety monitoring of herbal medicines in pharmacovigilance systems. 2004. https://www.who.int/publications/i/item/9241592214. Accessed 12 Aug 2024.
-
- Cañigueral S, Arruda Frommenwiler D, Reich E, Vila R. 7. High performance thin-layer chromatography (HPTLC) in the quality control of herbal products. In: India Recent Advances in Pharmaceutical Sciences VIII. 2018. pp. 119–36.
-
- EDQM (European Directorate for the Quality of Medicines and Health Care). European pharmacopoeia. 9 Th ed. Council of Europe, Strasbourg; 2018.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

