Storage stability of cowpea mosaic virus cancer drug candidate - a two-year update
- PMID: 40782045
- PMCID: PMC12490393
- DOI: 10.1080/17435889.2025.2544522
Storage stability of cowpea mosaic virus cancer drug candidate - a two-year update
Abstract
Aim: To evaluate the structural stability and biological activity of cowpea mosaic virus (CPMV) after 2 years of storage.
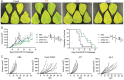
Materials and methods: CPMV was stored at room temperature (RT), 4°C, and -20°C for 2 years. Structural stability was assayed using dynamic light scattering (DLS), size exclusion chromatography (SEC), native and denaturing gel electrophoresis, and transmission electron microscopy (TEM). Biological activity was assessed by anti-tumor efficacy studies using a B16F10 dermal mouse melanoma model and by a cowpea plant infection challenge.
Results: Storage at -20°C preserved CPMV's structural properties and biological activity. Storage at 4°C caused aggregation, S-protein cleavage, and RNA degradation - samples stored at 4°C had decreased anti-tumor efficacy yet retained plant infectivity. CPMV stored at RT was degraded and lost biological activity.
Conclusion: Frozen storage allows CPMV to maintain structural and biological stability for at least 2 years.
Keywords: Cowpea mosaic virus; cancer immunotherapy; intratumoral immunotherapy; storage stability; translational drug development.
Conflict of interest statement
The authors declare the following competing financial interest(s): Dr Steinmetz is a co-founder of, has equity in, and has a financial interest in PlantiosX Inc. Dr Steinmetz is a co-founder of, has equity in, and has a financial interest in Mosaic ImmunoEngineering Inc. Dr Steinmetz is a co-founder and manager of Pokometz Scientific LLC, under which she is a paid consultant to Flagship Labs 95 Inc.
The other authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures

References
-
- Langley J, Pastural E, Halperin S, et al. A randomized controlled study to evaluate the safety and reactogenicity of a novel rVLP-based plant virus nanoparticle adjuvant combined with seasonal trivalent influenza vaccine following single immunization in healthy adults 18–50 years of age. Vaccines (Basel). 2020;8(3):393. doi: 10.3390/vaccines8030393 - DOI - PMC - PubMed
-
- Mao C, Beiss V, Fields J, et al. Cowpea mosaic virus stimulates antitumor immunity through recognition by multiple MYD88-dependent toll-like receptors. Biomaterials. 2021. Aug 01;275:120914. doi: 10.1016/j.biomaterials.2021.120914 - DOI - PMC - PubMed
-
•• This work provides the key mechanism data detailing that CPMV acts as TLR agonist.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
