Sex differences in resilience at puberty depend upon divergent effects of a stress steroid at α4βδ GABAA receptors
- PMID: 40782954
- PMCID: PMC12445324
- DOI: 10.1016/j.brainres.2025.149874
Sex differences in resilience at puberty depend upon divergent effects of a stress steroid at α4βδ GABAA receptors
Abstract
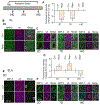
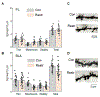
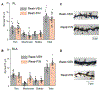
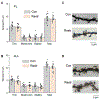
Resilience is a critical skill that lessens the adverse effects of stress which are increasingly reported in adolescents, where sex differences are noted. It is not known how this process develops in adolescence when unique changes in neuronal properties occur. For this study, pubertal mice were tested for their coping ability following 2-week restraint. We show here that this predictable stress produced resilience in pubertal female mice where time immobile in the forced swim test (FST) decreased by ∼50 % (P < 0.0001), an effect that extended into adulthood, and increased escape behavior 8-fold (P = 0.01). This effect was not seen in pubertal male or adult female mice. This process required the stress steroid 3α-OH,5α-pregnan-20-one (THP, allopregnanolone) and its primary target, α4βδ GABAA receptors (GABARs). These receptors emerge at puberty in prelimbic prefrontal cortex (PL PFC) and basolateral amygdala (BLA), which play a pivotal role in resilient behavior. Stress-induced release of THP decreased anxiety-like behavior (increasing open arm time in the elevated plus maze) and enhanced PFC-dependent learning (temporal order recognition) after 1d restraint in pubertal female, but not male, mice while differentially altering α4βδ expression in PL and BLA. This divergent THP-induced effect ultimately increased and decreased mushroom spine density in PL and BLA, respectively, to produce a circuit optimized for resilient behavior in the pubertal females. These findings demonstrate a novel mechanism for the development of resilience unique to the pubertal period. The results from the present study may suggest therapeutic strategies for adolescent stress which would impact mental health in adulthood.
Keywords: Adolescence; Allopregnanolone; Alpha-4; GABA(A) receptor; Prefrontal cortex; Resilience; Stress.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Amat J, Paul E, Zarza C, Watkins LR, Maier SF, 2006. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J. Neurosci 26, 13264–13272. 10.1523/JNEUROSCI.3630-06.2006. - DOI - PMC - PubMed
-
- Aoki C, Sabaliauskas N, Chowdhury T, Min JY, Colacino AR, Laurino K, et al. , 2012. Adolescent female rats exhibiting activity-based anorexia express elevated levels of GABA (A) receptor alpha4 and delta subunits at the plasma membrane of hippocampal CA1 spines. Synapse 66, 391–407. 10.1002/syn.21528. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

