F-actin disassembly by the oxidoreductase MICAL1 promotes mechano-dependent VWF-GPIbα interaction in platelets
- PMID: 40783397
- PMCID: PMC12335590
- DOI: 10.1038/s41467-025-62487-2
F-actin disassembly by the oxidoreductase MICAL1 promotes mechano-dependent VWF-GPIbα interaction in platelets
Abstract
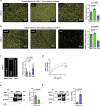
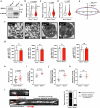
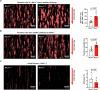
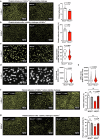
Mechano-dependent interactions are key to thrombus formation and hemostasis, enabling stable platelet adhesion to injured vessels. The interaction between von Willebrand factor (VWF) and the platelet receptor GPIb-IX-V is central to this process. While GPIbα connects to the actin cytoskeleton, whether actin dynamics are important for GPIbα function under hemodynamic, high shear conditions remains largely unknown. Here, we show that actin disassembly is critical for proper VWF-GPIbα binding under shear. Mechanistically, we identify the oxidoreductase MICAL1 as a shear-activated regulator that promotes local F-actin disassembly around the GPIb-IX-V complex. This enables its translocation to lipid rafts and reinforces VWF binding. MICAL1-deficient platelets display impaired adhesion, increased deformability under shear, and defective thrombus formation in vivo. Thus, MICAL1 drives shear-dependent actin remodeling that supports GPIb-IX-V mechanotransduction and platelet function. These findings uncover a role for actin oxidation in platelet adhesion, providing a connection between cytoskeletal redox control and platelet function during thrombus formation.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Dopheide, S. M., Maxwell, M. J. & Jackson, S. P. Shear-dependent tether formation during platelet translocation on von Willebrand factor. Blood99, 159–167 (2002). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

