Critical risks of haemoadsorption for COVID-19 patients and directions for future evaluations: a nationwide propensity score matched cohort study
- PMID: 40783423
- PMCID: PMC12335523
- DOI: 10.1038/s41598-025-13860-0
Critical risks of haemoadsorption for COVID-19 patients and directions for future evaluations: a nationwide propensity score matched cohort study
Abstract
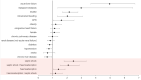
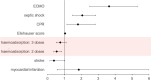
Haemoadsorption has been suggested as treatment adjunct for sepsis and septic shock, cardiac surgery, acute respiratory distress syndrome, and coronavirus disease 2019 (COVID-19). Randomised clinical trials did not provide conclusive evidence for benefits and even suggest risks in COVID-19 patients. Retrospective observational cohort study based on hospital remuneration data from all COVID-19 patients treated in intensive care units in Germany between 01/01/2020 and 12/31/2021. Regression modelling was performed for 1:1 propensity score matching of 2058 patients. Two-sided probability values for group comparisons and regression models with spline functions controlling for non-linear relationships and medically relevant interaction variables were calculated. In-hospital mortality of patients supported with haemoadsorption was significantly higher compared to matched control patients (74.6% vs. 70.3%, p = 0.0299). Haemoadsorption was associated with coagulopathy (68.0% vs. 54.9%, p < 0.0001), cardiac arrhythmia (49.2% vs. 44.2%, p = 0.0272), and cardiopulmonary resuscitation (CPR, 19.3% vs. 13.1%, p = 0.0002). Further, haemoadsorption increased the chance of death for COVID-19 patients without septic shock (odds ratio, OR [within a 95% confidence interval, CI]; 1.40 [1.05-1.86]) and did not improve survival of septic shock patients (1.19 [0.85-1.67]). Independent variables with a significant impact on mortality included the use of extracorporeal membrane oxygenation (ECMO, 2.15 [1.68-2.76]) and CPR (1.60 [1.03-2.45]). The timing of the haemoadsorption therapy had no effect on patients´ outcomes. Due to inconclusive evidence for benefit and potential harm, haemoadsorption therapy should be limited to thoroughly designed clinical trials before introduced into clinical routine in the context of COVID-19.
Keywords: Acute respiratory distress syndrome; COVID-19; Cytokine adsorption; Haemoadsorption; Hyperinflammation; In-hospital mortality; Intensive care unit; Septic shock.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The Department of Anaesthesiology, Intensive Care Medicine & Pain Therapy of the University Hospital Frankfurt, Goethe University received support from B. Braun Melsungen, CSL Behring, Fresenius Kabi, and Vifor Pharma for the implementation of Frankfurt‘s Patient Blood Management program.KZ has received honoraria for participation in advisory board meetings for Haemonetics and Vifor and received speaker fees from CSL Behring, Masimo, Pharmacosmos, Boston Scientific, Salus, iSEP, Edwards, Hemosonics and GE Healthcare. He is the Principal Investigator of the EU-Horizon 2020 project ENVISION (Intelligent plug-and-play digital tool for real-time surveillance of COVID-19 patients and smart decision-making in Intensive Care Units) and Horizon Europe 2021 project COVend (Biomarker and AI-supported FX06 therapy to prevent progression from mild and moderate to severe stages of COVID-19). Partner for EU Horizon 2023 project EDiHTA. J.K. and B.F. are Deputy Principal Investigator of ENVISION and COVend. A.S. and P.M. reports travel support, lecture honoraria (C.S.) and research funding from CytoSorbents Europe and lecture honoraria from Getinge. All other authors have no competing interests. Ethical approval and consent to participate: All data processing was performed in accordance with the Declaration of Helsinki. According to § 21KHEntgG the reimbursement data is available for scientific use. Due to the institutional anonymization and retrospective nature of the study, no conclusions can be drawn at the individual case level. For this reason, the Ethics Committee of University Hospital Frankfurt waived the requirement for ethical approval and the need to obtain informed consent for this study (Chair: Prof. Dr. Harder, Ref: 2022–766). Consent for publication: As these are anonymised register data, no consensus of the patients can be collected.
Figures

Similar articles
-
Extracorporeal carbon dioxide removal for the treatment of acute hypoxaemic respiratory failure: the REST RCT.Health Technol Assess. 2025 Jul;29(33):1-16. doi: 10.3310/GJDM0320. Health Technol Assess. 2025 Jul;29(33):1-16. doi: 10.3310/GJDM0320. PMID: 40758387 Clinical Trial.
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
-
Remdesivir for the treatment of COVID-19.Cochrane Database Syst Rev. 2023 Jan 25;1(1):CD014962. doi: 10.1002/14651858.CD014962.pub2. Cochrane Database Syst Rev. 2023. PMID: 36695483 Free PMC article.
-
Systemic Inflammatory Response Syndrome.2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31613449 Free Books & Documents.
-
Colchicine for the treatment of COVID-19.Cochrane Database Syst Rev. 2021 Oct 18;10(10):CD015045. doi: 10.1002/14651858.CD015045. Cochrane Database Syst Rev. 2021. PMID: 34658014 Free PMC article.
References
-
- Supady, A., Brodie, D. & Wengenmayer, T. Extracorporeal haemoadsorption: does the evidence support its routine use in critical care? Lancet Respir Med.10, 307–312 (2022). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

