Pharmacokinetic, biodistribution, safety and efficacy studies of borophenylalanine (BPA) in BNCT in hepatocellular carcinoma cells and tumor-bearing mouse model
- PMID: 40783437
- PMCID: PMC12335535
- DOI: 10.1038/s41598-025-14885-1
Pharmacokinetic, biodistribution, safety and efficacy studies of borophenylalanine (BPA) in BNCT in hepatocellular carcinoma cells and tumor-bearing mouse model
Abstract
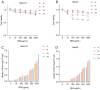
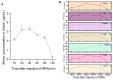
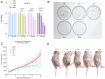
Boron neutron capture therapy (BNCT), using boronophenylalanine (BPA) as the main boron carrier, is a dual-targeted particle radiotherapy at the cellular level. Although BPA shows clinical promise in liver cancer, there is relatively little basic research on its effect on hepatocellular carcinoma. Therefore, we systematically evaluated the uptake, safety, pharmacokinetics, and therapeutic efficacy of BPA. Boron uptake in hepatocellular carcinoma cells (Hepa1-6, HepG2) was quantified by ICP-AES, revealing concentration- and time-dependent accumulation (plateau at 6 h), while CCK-8 assays indicated significant cytotoxicity at 24 h. Pharmacokinetic studies in Sprague-Dawley (SD) rats showed rapid boron distribution (peak at 25 ± 5.8 min) with a blood clearance half-life of 74.71 ± 52.22 min. In tumor-bearing mouse models, BPA achieved tumor-specific targeting, with tumor-to-normal tissue (T/N) and tumor-to-blood (T/B) ratios exceeding 2 and 4, respectively, at 2 h post-injection, followed by rapid systemic clearance. Cell viability significantly decreased after BPA-BNCT irradiation, and the tumor growth inhibition rate in mice reached 77%. BPA did not produce tissue damage in vivo, and there were no abnormalities in blood counts or liver or kidney function in vivo after irradiation. These findings suggest that BPA can be selectively enriched in hepatocellular tumors with good pharmacokinetics and therapeutic efficacy, supporting its clinical application in BNCT of hepatocellular carcinoma.
Keywords: BPA; Boron neutron capture therapy; Distribution of drugs; Pharmacokinetic.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Institutional review board statement: The animal study protocol was approved by The Committee on the Ethics of Animal Experiments of Lanzhou University First Hospital (LDYYLL2024-684). We used isoflurane inhalation anesthetic to euthanize the experimental mice.
Figures




Similar articles
-
Suitability of boron carriers for BNCT: accumulation of boron in malignant and normal liver cells after treatment with BPA, BSH and BA.Appl Radiat Isot. 2009 Jul;67(7-8 Suppl):S105-8. doi: 10.1016/j.apradiso.2009.03.025. Epub 2009 Mar 26. Appl Radiat Isot. 2009. PMID: 19375330
-
Boron-Containing Mesoporous Silica Nanoparticles with Effective Delivery and Targeting of Liver Cancer Cells for Boron Neutron Capture Therapy.ACS Appl Mater Interfaces. 2024 May 8;16(18):22934-22945. doi: 10.1021/acsami.4c02897. Epub 2024 Apr 30. ACS Appl Mater Interfaces. 2024. PMID: 38686647
-
Exploratory Rat Toxicology and Efficacy of the Boron Neutron Capture Therapy Drug Boronotyrosine in a Mouse Model of Head and Neck Cancer.Int J Radiat Oncol Biol Phys. 2025 Jul 28:S0360-3016(25)06030-4. doi: 10.1016/j.ijrobp.2025.07.1432. Online ahead of print. Int J Radiat Oncol Biol Phys. 2025. PMID: 40738349
-
Exploring Targeted Delivery Systems for Boron Neutron Capture Therapy and Its Potential as a Promising Therapeutic Modality for Hepatocellular Carcinoma.Cancer Biother Radiopharm. 2025 Aug 25. doi: 10.1177/10849785251370873. Online ahead of print. Cancer Biother Radiopharm. 2025. PMID: 40854589 Review.
-
Ablative and non-surgical therapies for early and very early hepatocellular carcinoma: a systematic review and network meta-analysis.Health Technol Assess. 2023 Dec;27(29):1-172. doi: 10.3310/GK5221. Health Technol Assess. 2023. PMID: 38149643 Free PMC article.
References
-
- Ferlay, J. et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer. 144, 1941–1953 (2019). - PubMed
-
- Chen, S., Cao, Q., Wen, W. & Wang, H. Targeted therapy for hepatocellular carcinoma: challenges and opportunities. Cancer Lett.460, 1–9 (2019). - PubMed
-
- Suzuki, M. et al. First attempt of Boron neutron capture therapy (BNCT) for hepatocellular carcinoma. Jpn J. Clin. Oncol.37, 376–381 (2007). - PubMed
-
- Yura, Y. & Fujita, Y. Boron neutron capture therapy as a novel modality of radiotherapy for oral cancer: principle and antitumor effect. Oral Sci. Int.10, 9–14 (2013).
-
- Zhang, H., Zhang, P., Zhu, Y. & Ran, J. Clinical research progress of Boron neutron capture therapy. Chin. J. Radiation Oncol.32, 848–853 (2023).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

