Synthesis of heterocycle based carboxymethyl cellulose conjugates as novel anticancer agents targeting HCT116, MCF7, PC3 and A549 cells
- PMID: 40783442
- PMCID: PMC12335502
- DOI: 10.1038/s41598-025-14146-1
Synthesis of heterocycle based carboxymethyl cellulose conjugates as novel anticancer agents targeting HCT116, MCF7, PC3 and A549 cells
Abstract
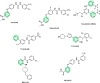
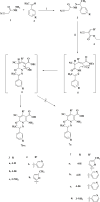
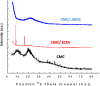
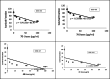
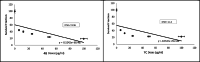
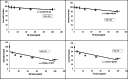
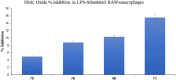
Toward developing anticancer agents, heterocycle-based carboxymethyl cellulose conjugates have been synthesized. 2-Cyano-N'-(aryl/heteroarylethylidene)acetohydrazides and ethyl 2-cyano-3-(heteryl)acrylates were utilized as precursors for the synthesis of pyridine-based compounds. The chemical structures of the synthesized derivatives were characterized using various spectroscopic techniques, including 1H-, 13C-NMR, Fourier transform infrared spectroscopy (FTIR), as well as scanning electron microscopy (SEM).The anticancer effects of compounds on HCT-116, MCF-7, PC3 and A549 cancer cell lines were investigated and their cytotoxicity against RPE-1 normal cells was estimated to determine their safety. Compounds 4b and 7c exhibit high selectivity toward cancer cells while maintaining a strong safety margin for normal cells. The results demonstrated that the novel heterocycle-based carboxymethyl cellulose conjugates are promising and can be further evaluated as a potential therapeutic agent.
Keywords: Anticancer; Carboxymethyl cellulose; Pyridine; Synthesis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval: The study was approved by the Medical Research Ethics Committee (MERC) fedral (accurance no. : FWA 00014747).
Figures




References
-
- Manaithiya, A. et al. Current status of novel pyridine fused derivatives as anticancer agents: An insight into future perspectives and structure activity relationship (SAR). Curr. Top. Med. Chem.21 (25), 2292–2349 (2021). - PubMed
-
- Prachayasittikul, S. et al. Roles of pyridine and pyrimidine derivatives as privileged scaffolds in anticancer agents. Mini Rev. Med. Chem.17 (10), 869–901 (2017). - PubMed
-
- Henry, G. D. De Novo synthesis of substituted pyridines. Tetrahedron29 (60), 6043–6061 (2004).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

