Supporting intraoperative margin assessment using deep learning for automatic tumour segmentation in breast lumpectomy micro-PET-CT
- PMID: 40783490
- PMCID: PMC12335567
- DOI: 10.1038/s41523-025-00797-w
Supporting intraoperative margin assessment using deep learning for automatic tumour segmentation in breast lumpectomy micro-PET-CT
Abstract
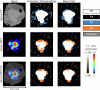
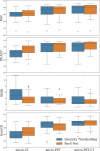
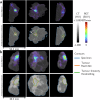
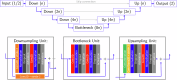
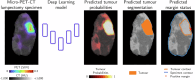
Complete tumour removal is vital in curative breast cancer (BCa) surgery to prevent recurrence. Recently, [18F]FDG micro-PET-CT of lumpectomy specimens has shown promise for intraoperative margin assessment (IMA). To aid interpretation, we trained a 2D Residual U-Net to delineate invasive carcinoma of no special type in micro-PET-CT lumpectomy images. We collected 53 BCa lamella images from 19 patients with true histopathology-defined tumour segmentations. Group five-fold cross-validation yielded a dice similarity coefficient of 0.71 ± 0.20 for segmentation. Afterwards, an ensemble model was generated to segment tumours and predict margin status. Comparing predicted and true histopathological margin status in a separate set of 31 micro-PET-CT lumpectomy images of 31 patients achieved an F1 score of 84%, closely matching the mean performance of seven physicians who manually interpreted the same images. This model represents an important step towards a decision-support system that enhances micro-PET-CT-based IMA in BCa, facilitating its clinical adoption.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: L.M. is a research engineer at XEOS Medical, and V.K. is a shareholder and board member of XEOS Medical. M.G., K.D.M., B.V.d.B., S.V.H., K.V.d.V., and C.V. declare that they have no competing interests.
Figures

Similar articles
-
123I-MIBG scintigraphy and 18F-FDG-PET imaging for diagnosing neuroblastoma.Cochrane Database Syst Rev. 2015 Sep 29;2015(9):CD009263. doi: 10.1002/14651858.CD009263.pub2. Cochrane Database Syst Rev. 2015. PMID: 26417712 Free PMC article.
-
PET-CT for assessing mediastinal lymph node involvement in patients with suspected resectable non-small cell lung cancer.Cochrane Database Syst Rev. 2014 Nov 13;2014(11):CD009519. doi: 10.1002/14651858.CD009519.pub2. Cochrane Database Syst Rev. 2014. PMID: 25393718 Free PMC article.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
The value of FDG positron emission tomography/computerised tomography (PET/CT) in pre-operative staging of colorectal cancer: a systematic review and economic evaluation.Health Technol Assess. 2011 Sep;15(35):1-192, iii-iv. doi: 10.3310/hta15350. Health Technol Assess. 2011. PMID: 21958472 Free PMC article.
-
Development and Validation of a Convolutional Neural Network Model to Predict a Pathologic Fracture in the Proximal Femur Using Abdomen and Pelvis CT Images of Patients With Advanced Cancer.Clin Orthop Relat Res. 2023 Nov 1;481(11):2247-2256. doi: 10.1097/CORR.0000000000002771. Epub 2023 Aug 23. Clin Orthop Relat Res. 2023. PMID: 37615504 Free PMC article.
References
-
- Cardoso, F. et al. Early breast cancer: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol.30, 1194–1220 (2019). - PubMed
-
- McDonald, E. S., Clark, A. S., Tchou, J., Zhang, P. & Freedman, G. M. Clinical diagnosis and management of breast cancer. J. Nucl. Med.57, 9S–16S (2016). - PubMed
-
- Brouwer de Koning, S. G., Vrancken Peeters, M.-J. T. F. D., Jóźwiak, K., Bhairosing, P. A. & Ruers, T. J. M. Tumor resection margin definitions in breast-conserving surgery: systematic review and meta-analysis of the current literature. Clin. Breast Cancer18, e595–e600 (2018). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

