Circulating Hsp70: a tumor biomarker for lymph node metastases and early relapse in thoracic cancer
- PMID: 40783506
- PMCID: PMC12335804
- DOI: 10.1186/s12885-025-14725-5
Circulating Hsp70: a tumor biomarker for lymph node metastases and early relapse in thoracic cancer
Abstract
Background: Heat shock protein 70 (Hsp70) which is frequently overexpressed in many different cancer types is also present on the plasma membrane of tumor but not normal cells. The intensity of membrane-expressed Hsp70 (mHsp70) is associated with disease progression and treatment resistance. It has also been shown that Hsp70 can be actively released into the circulation by mHsp70 positive, viable tumor cells in the form of extracellular lipid microvesicles expressing mHsp70, the levels of which might therefore act as a potential biomarker for tumor aggressiveness in lung malignancies.
Methods: Extracellular Hsp70 (eHsp70) was measured in the plasma of patients with non-small cell lung cancer (n = 178, NSCLC) and lung metastases of extrathoracic tumors (n = 35) prior to surgery using the Hsp70-exo ELISA which detects microvesicle-associated eHsp70 and the patient`s immunophenotype was determined by flow cytometric analysis of the corresponding peripheral blood lymphocytes.
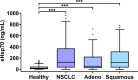
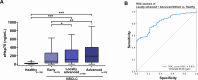
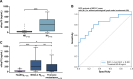
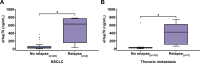
Results: eHsp70 values were significantly higher in patients with NSCLC than in healthy individuals, with no differences between adeno and squamous cell carcinomas. Levels of circulating eHsp70 which are associated with the Programmed cell death protein 1 (PD-L1) status, gradually increased from early stage to metastatic disease, and patients with lymph node metastases in surgically treatable NSCLC had significantly higher eHsp70 levels than nodal negative patients. In all tumor stages, total lymphocyte counts were significantly reduced and immunoregulatory T (Treg) cell counts were increased compared to healthy controls. Lower CD4 + T helper cell and higher CD3-/CD56+/CD94+/CD69+/NKp30+/NKp46 + NK cell ratios were only found in patients with thoracic metastases of other primary tumors. An early relapse after complete resection with curative intent correlated with significantly elevated eHsp70 levels which were measured prior to surgery, in all thoracic cancer patients.
Conclusions: In summary, we propose circulating eHsp70 levels before any treatment as a predictive biomarker for the presence of lymph node metastases and early therapy failure in patients with thoracic malignancies.
Keywords: Biomarker; Extracellular Hsp70; Immune-phenotype; Liquid biopsy; NSCLC.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the local ethics committee of the TUM School of Medicine and Health (ethical approval number: 2403/09 and 2428/09), and written informed consent was obtained from all patients and healthy donors before start of the study. Blood sampling was conducted in accordance to the Declaration of Helsinki. Consent for publication: Not applicable. Competing interests: AGP is CEO and GM is CSO of multimmune GmbH. A granted US patent protects the Hsp70-exo ELISA for determining free and vesicular eHsp70 in liquid biopsies. All other authors [DL, NT, VM, SS (Seier), JB, ER, AD, SS (Safi)] have no known competing interests.
Figures

Similar articles
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Can a Liquid Biopsy Detect Circulating Tumor DNA With Low-passage Whole-genome Sequencing in Patients With a Sarcoma? A Pilot Evaluation.Clin Orthop Relat Res. 2025 Jan 1;483(1):39-48. doi: 10.1097/CORR.0000000000003161. Epub 2024 Jun 21. Clin Orthop Relat Res. 2025. PMID: 38905450
-
PET-CT for assessing mediastinal lymph node involvement in patients with suspected resectable non-small cell lung cancer.Cochrane Database Syst Rev. 2014 Nov 13;2014(11):CD009519. doi: 10.1002/14651858.CD009519.pub2. Cochrane Database Syst Rev. 2014. PMID: 25393718 Free PMC article.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
References
-
- (WHO) WHO. Lung cancer. 2023. https://www.who.int/news-room/fact-sheets/detail/lung-cancer
-
- Robert Koch Institute (ed) and the Association of Population-based Cancer Registries in Germany (ed) Berlin. Cancer in Germany 2019/2020 14th edition.
-
- Kocher F, Hilbe W, Seeber A, Pircher A, Schmid T, Greil R, et al. Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer. 2015;87(2):193–200. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

