Multidimensional comparative evaluation of first-line therapies for extensive-stage small cell lung cancer: a systematic review and network meta-analysis of clinical efficacy and safety profiles
- PMID: 40783512
- PMCID: PMC12335103
- DOI: 10.1186/s12885-025-14750-4
Multidimensional comparative evaluation of first-line therapies for extensive-stage small cell lung cancer: a systematic review and network meta-analysis of clinical efficacy and safety profiles
Abstract
Background: The first-line treatment for extensive-stage small cell lung cancer (ES-SCLC) has evolved from chemotherapy alone to chemoimmunotherapy. However, the improvements in overall survival (OS) and progression-free survival (PFS) have been modest. Therefore, this study employs a comprehensive multidimensional evaluation framework to identify optimized therapeutic combinations with enhanced efficacy and improved safety profiles in the immunotherapy era.
Methods: An adaptive search strategy was employed to retrieve all relevant literature from electronic databases, including PubMed, Embase, Web of Science, and the Cochrane Library, from database inception to November 2024. The retrieved studies were carefully screened according to pre-designed inclusion and exclusion criteria. Clinical research articles and their supplementary materials that met the criteria were obtained and thoroughly reviewed. Manual data extraction was conducted, with the safety data and efficacy outcomes. A network meta-analysis of all acquired data for each outcome was performed. The study protocol was pre-registered in PROSPERO, CRD42024612944.
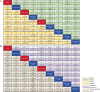
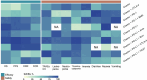
Results: This network meta-analysis included 6,473 patients from 14 head-to-head randomized controlled trials (RCTs). Compared with etoposide-platinum chemotherapy combined with a programmed cell death ligand 1 inhibitor (the Chemo + PD-L1 regimen), the addition of anlotinib (the Chemo + PD-L1 + Anlo regimen) resulted in better PFS (hazard ratio (HR), 0.42; 95% confidence interval (CI), 0.33-0.54) and objective response rate (ORR) (odds ratio (OR), 1.81; 95% CI, 1.13-2.91). Moreover, adding BMS-986012 (anti-fucosyl-GM1 antibodies) to the Chemo + PD-L1 regimen ranked first in the surface under the cumulative ranking curve (SUCRA, 0.96) analysis for OS. Compared with the Chemo + PD-L1 regimen, the addition of an anti-CTLA-4 inhibitor (the Chemo + PD-L1 + CTLA-4 regimen) was associated with an increased risk of treatment-related adverse events (TRAEs) of grades ≥ 3 (risk ratio (RR), 1.19; 95%CI, 1.04-1.36).
Conclusions: Incorporating anlotinib into the Chemo + PD-L1 regimen can be a viable first-line option for patients with high tumor burden, but cannot fully replace the current first-line standard-of-care (SOC). Chemoimmunotherapy combined with immune-related targeting drugs demonstrates the potential to improve overall survival.
Keywords: Angiogenesis; Chemoimmunotherapy; Extensive-stage; Immune checkpoint inhibitor; Network meta-analysis; Small cell lung cancer.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Efficacy and safety of first-line PD-1/PD-L1 inhibitors combined with or without anti-angiogenesis therapy for extensive-stage small-cell lung cancer: a network meta-analysis.Ther Adv Med Oncol. 2025 Jun 25;17:17588359251348310. doi: 10.1177/17588359251348310. eCollection 2025. Ther Adv Med Oncol. 2025. PMID: 40574965 Free PMC article.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Comparison of Efficacy and Safety of Single and Double Immune Checkpoint Inhibitor-Based First-Line Treatments for Advanced Driver-Gene Wild-Type Non-Small Cell Lung Cancer: A Systematic Review and Network Meta-Analysis.Front Immunol. 2021 Aug 16;12:731546. doi: 10.3389/fimmu.2021.731546. eCollection 2021. Front Immunol. 2021. PMID: 34484242 Free PMC article.
-
Comparison of efficacy and safety of PD-1/PD-L1 combination therapy in first-line treatment of advanced NSCLC: an updated systematic review and network meta-analysis.Clin Transl Oncol. 2024 Oct;26(10):2488-2502. doi: 10.1007/s12094-024-03442-3. Epub 2024 Apr 16. Clin Transl Oncol. 2024. PMID: 38625495
-
Efficacy and safety of immunotherapy combined with chemotherapy in patients with ES-SCLC: A systematic review and network meta-analysis of RCTs and RWSs.Thorac Cancer. 2024 Nov;15(33):2375-2385. doi: 10.1111/1759-7714.15458. Epub 2024 Oct 13. Thorac Cancer. 2024. PMID: 39397360 Free PMC article.
References
-
- Murray N, Coy P, Pater JL, Hodson I, Arnold A, Zee BC, Payne D, Kostashuk EC, Evans WK, Dixon P, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National cancer Institute of Canada clinical trials group. J Clin Oncol. 1993;11(2):336–44. - PubMed
-
- Wang J, Zhou C, Yao W, Wang Q, Min X, Chen G, Xu X, Li X, Xu F, Fang Y, et al. Adebrelimab or placebo plus carboplatin and Etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(6):739–47. - PubMed
-
- Megyesfalvi Z, Gay CM, Popper H, Pirker R, Ostoros G, Heeke S, Lang C, Hoetzenecker K, Schwendenwein A, Boettiger K, et al. Clinical insights into small cell lung cancer: tumor heterogeneity, diagnosis, therapy, and future directions. Cancer J Clin. 2023;73(6):620–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2022YFC2404605/National Key Research and Development Program
- WKZX2023Cx020012/Post-Marketing Clinical Research Special Project on Innovative Drugs
- 82172865/National Natural Science Foundation of China
- ZR2023LZL002/Natural Science Foundation of Shandong Province
- ZR2024MH007/Natural Science Foundation of Shandong Province
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

