Inhibition of astrocyte signaling leads to sex-specific changes in microglia phenotypes in a diet-based model of cerebral small vessel disease
- PMID: 40783543
- PMCID: PMC12335036
- DOI: 10.1186/s12974-025-03523-2
Inhibition of astrocyte signaling leads to sex-specific changes in microglia phenotypes in a diet-based model of cerebral small vessel disease
Abstract
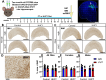
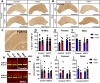
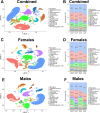
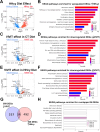
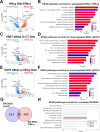
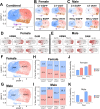
Hyperhomocysteinemia (HHcy)-inducing diets recapitulate cerebral small vessel disease phenotypes in mice including cerebrovascular pathology/dysfunction, neuroinflammation, synaptic deficits, and cognitive decline. We recently showed that astrocyte signaling through calcineurin(CN)/nuclear factor of activated T cells (NFATs) plays a causative role in these phenotypes. Here, we assessed the impact of astrocytic signaling on microglia, which set the inflammatory tone in brain. Seven-to-eight-week-old male and female C57BL/6 J mice received intrahippocampal injections of adeno-associated virus (AAV) expressing EGFP (AAV2/5-Gfa2-EGFP) or AAV expressing the NFAT inhibitor VIVIT (i.e., AAV2/5-Gfa2-VIVIT-EGFP). Mice were then fed with control chow (CT) or B-vitamin-deficient chow for 12 weeks to induce HHcy. Immunohistochemistry and Western blot analyses suggested that expression of the homeostatic microglial marker, P2RY12, responded differently to AAV treatments depending on diet and sex. We next conducted single-cell RNA sequencing (scRNA-seq) to determine if microglial genes and/or clustering patterns were differentially sensitive to diet and AAV, depending on sex. In males, disease-associated microglial genes and subclusters were overrepresented in HHcy-treated mice, while VIVIT promoted the appearance of homeostatic microglial genes and clusters. In contrast, microglial genes in females were less sensitive to diet and AAV treatments, though disease-like patterns were also observed in the HHcy condition. Very few of the HHcy-sensitive microglial genes in females were affected by VIVIT. Though based on small sample sizes, the results suggest a sexually dimorphic influence of astrocyte signaling on microglial transcriptional phenotypes in the context of HHcy and small cerebral vessel disease. However, these interpretations will need to be bolstered with additional biological replicates and more stringent statistical analyses.
Keywords: Astrocyte reactivity; Calcium; Microglia; Neuroinflammation; Vascular.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by University of Kentucky Institutional Animal Care and Use Committees. Consent for publication: NA. Competing interests: The authors declare no competing interests.
Figures
Update of
-
Inhibition of astrocyte signaling leads to sex-specific changes in microglia phenotypes in a diet-based model of small cerebral vessel disease.Res Sq [Preprint]. 2025 Mar 17:rs.3.rs-6198453. doi: 10.21203/rs.3.rs-6198453/v1. Res Sq. 2025. Update in: J Neuroinflammation. 2025 Aug 9;22(1):202. doi: 10.1186/s12974-025-03523-2. PMID: 40166012 Free PMC article. Updated. Preprint.
Similar articles
-
Inhibition of astrocyte signaling leads to sex-specific changes in microglia phenotypes in a diet-based model of small cerebral vessel disease.Res Sq [Preprint]. 2025 Mar 17:rs.3.rs-6198453. doi: 10.21203/rs.3.rs-6198453/v1. Res Sq. 2025. Update in: J Neuroinflammation. 2025 Aug 9;22(1):202. doi: 10.1186/s12974-025-03523-2. PMID: 40166012 Free PMC article. Updated. Preprint.
-
Hyperhomocysteinemia-induced VCID results in visual deficits, reduced neuroinflammation and vascular alterations in the retina.J Neuroinflammation. 2025 Jan 30;22(1):23. doi: 10.1186/s12974-025-03332-7. J Neuroinflammation. 2025. PMID: 39885592 Free PMC article.
-
Targeting Astrocyte Signaling Alleviates Cerebrovascular and Synaptic Function Deficits in a Diet-Based Mouse Model of Small Cerebral Vessel Disease.J Neurosci. 2023 Mar 8;43(10):1797-1813. doi: 10.1523/JNEUROSCI.1333-22.2023. Epub 2023 Feb 6. J Neurosci. 2023. PMID: 36746627 Free PMC article.
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
-
Antithrombotic therapy to prevent cognitive decline in people with small vessel disease on neuroimaging but without dementia.Cochrane Database Syst Rev. 2022 Jul 14;7(7):CD012269. doi: 10.1002/14651858.CD012269.pub2. Cochrane Database Syst Rev. 2022. PMID: 35833913 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

