Isolation of kinetically-stabilised diarylchalcogenide radical cations
- PMID: 40783648
- PMCID: PMC12335450
- DOI: 10.1038/s42004-025-01613-z
Isolation of kinetically-stabilised diarylchalcogenide radical cations
Abstract
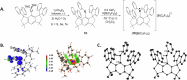
Chalcogenide radical cations [R2E]•+ (E = S, Se, Te) are commonly short-lived intermediates of fundamental interest. Sulfide radical cations in particular are associated in vivo with oxidative stress and neuropathological processes. Having succeeded in the preparation of meta-terphenyl-based dichalcogenide radical cations [R2E2]•+ (E = S, Se, Te), and a telluride analogue [R2Te]•+ in the past, we aimed to complete the series regarding sulfur and selenium. Here we report on the single-electron oxidation of diarylchalcogenides MSFluindPhE (E = S, Se, Te; MSFluind = dispiro[fluorene-9,3'-(1',1',7',7'-tetramethyl-s-hydrindacen-4'-yl)-5',9"-fluorene]) using XeF2 in the presence of K[B(C6F5)4], which afforded deeply coloured and isolable radical cation salts [MSFluindPhE][B(C6F5)4] (E = S, Se, Te). Structural and electronic properties were characterised by electron paramagnetic spectroscopy, cyclic voltammetry, optical absorption spectroscopy and single crystal X-ray diffraction (E = Se, Te), combined with extensive quantum mechanical computations.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Biological activities of optimized biosynthesized selenium nanoparticles using Proteus mirabilis PQ350419 alone or combined with chitosan and ampicillin against common multidrug-resistant bacteria.Microb Cell Fact. 2025 Jul 5;24(1):159. doi: 10.1186/s12934-025-02783-0. Microb Cell Fact. 2025. PMID: 40618114 Free PMC article.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Quasiparticle Band Structure, Exciton, and Optical Property in Janus Structures of Transition-Metal Dichalcogenide Monolayers.ACS Omega. 2025 Jul 11;10(28):30924-30934. doi: 10.1021/acsomega.5c03536. eCollection 2025 Jul 22. ACS Omega. 2025. PMID: 40727804 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis.Cochrane Database Syst Rev. 2017 Jun 22;6(6):CD007419. doi: 10.1002/14651858.CD007419.pub5. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2018 Oct 16;10:CD007419. doi: 10.1002/14651858.CD007419.pub6. PMID: 28639415 Free PMC article. Updated.
References
-
- Gomberg, M. Triphenylmethyl, ein Fall von dreiwerthigem Kohlenstoff. Ber. Dtsch. Chem. Ges.33, 3150–3163 (1900).
-
- Power, P. P. Persistent and stable radicals of the heavier main group elements and related species. Chem. Rev.103, 789–810 (2003). - PubMed
-
- Feng, Z., Tang, S., Su, Y. & Wang, X. Recent advances in stable main group element radicals: preparation and characterization. Chem. Soc. Rev.51, 5930–5973 (2022). - PubMed
-
- Glass, R. S. Sulfur radicals and their application. Top. Curr. Chem.376, 1–42 (2018). - PubMed
-
- Nishinaga, T. & Komatsu, K. Persistent pi radical cations: self-association and its steric control in the condensed phase. Org. Biomol. Chem.3, 561–569 (2005). - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

