Isoquercitrin mitigates intestinal ischemia-reperfusion injury by regulating intestinal flora and inhibiting NLRP3 inflammasome activation
- PMID: 40784044
- PMCID: PMC12357270
- DOI: 10.1016/j.redox.2025.103803
Isoquercitrin mitigates intestinal ischemia-reperfusion injury by regulating intestinal flora and inhibiting NLRP3 inflammasome activation
Abstract
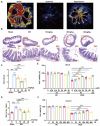
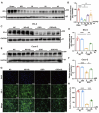
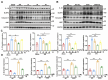
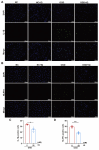
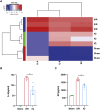
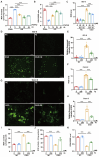
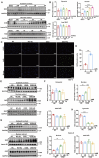
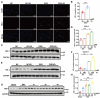
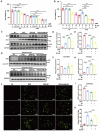
Intestinal ischemia-reperfusion (II/R) injury, frequently observed in clinical emergencies such as trauma, infection, and transplantation, leads to severe epithelial necrosis, loss of villi, and alarmingly high mortality rates (50 %-90 %), yet current pharmaceutical treatments largely prove ineffective. This study employs network pharmacology alongside in vivo and in vitro experiments to explore the potential of isoquercitrin, a flavonoid abundant in various dietary sources and known for its anti-inflammatory and antioxidant properties, in mitigating intestinal II/R injury. We found that isoquercitrin significantly reinforced the integrity of the intestinal barrier and markedly alleviated damage associated with II/R injury. Additionally, it enhanced the intestinal microbiota structure by promoting microbial diversity and supporting beneficial bacterial populations. According to network pharmacology analyses, isoquercitrin may prevent II/R injury by modulating redox-related pathways and regulating inflammatory responses mediated by the NLRP3 inflammasome. This protective effect is evidenced by reduced levels of reactive oxygen species (ROS) and malondialdehyde (MDA), as well as an increased GSH/GSSG ratio and enhanced superoxide dismutase (SOD) activity. Isoquercitrin also inhibited NLRP3 inflammasome activation and decreased the expression of downstream factors, including Caspase-1, IL-1β, IL-6, and keratinocyte-derived cytokine (KC). The observed effects correlate with enhancement of nuclear translocation of nuclear factor erythroid 2-related factor 2 (Nrf2) and increased expression of heme oxygenase-1 (HO-1) in a dose-dependent manner, and these beneficial effects were abolished by both ML385 (an Nrf2 inhibitor) and siNrf2. Thus, activating the Nrf2/HO-1 signaling pathway is crucial to isoquercitrin's protective role in intestinal II/R injury. The present findings underscore the therapeutic potential of isoquercitrin in managing intestinal II/R injury.
Keywords: Inflammatory cytokines; Intestinal flora; Intestinal ischemia-reperfusion; Isoquercitrin; Nrf2/HO-1.
Copyright © 2025 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Wen S., Li X., Ling Y., Chen S., Deng Q., Yang L., Li Y., Shen J., Qiu Y., Zhan Y., Lai H., Zhang X., Ke Z., Huang W. HMGB1-associated necroptosis and kupffer cells M1 polarization underlies remote liver injury induced by intestinal ischemia/reperfusion in rats. FASEB J. 2020;34(3):4384–4402. - PubMed
-
- Feng D., Yao J., Wang G., Li Z., Zu G., Li Y., Luo F., Ning S., Qasim W., Chen Z., Tian X. Inhibition of p66Shc-mediated mitochondrial apoptosis via targeting prolyl-isomerase Pin1 attenuates intestinal ischemia/reperfusion injury in rats. Clin. Sci. (Lond.) 2017;131(8):759–773. - PubMed
-
- Acosta S. Epidemiology of mesenteric vascular disease: clinical implications. Semin. Vasc. Surg. 2010;23(1):4–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

