Small molecule agonist TPC2-A1-N increases intracellular Ca2+ independent of two-pore channels
- PMID: 40784451
- PMCID: PMC12446622
- DOI: 10.1016/j.jbc.2025.110576
Small molecule agonist TPC2-A1-N increases intracellular Ca2+ independent of two-pore channels
Abstract
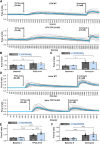
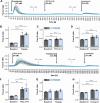
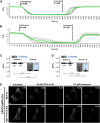
Two-pore channels (TPCs) constitute a small family of cation channels expressed in endo-lysosomal compartments. TPCs have been characterized as important constituents controlling Ca2+-mediated vesicular membrane fusion and fission, thereby regulating intracellular organelle trafficking. Two activators, nicotinic acid adenine dinucleotide phosphate and phosphatidylinositol-3,5-bisphosphate, induce ion flux through TPCs. The membrane-permeable small molecule activators TPC2-A1-N and TPC2-A1-P have been identified and postulated to mimic their action and to discriminate for a preferential selectivity either for Ca2+ or for Na+. This was observed only for TPC2 and was independent of nicotinic acid adenine dinucleotide phosphate-binding proteins. Here, we applied TPC2-A1-N and measured intracellular increase of Ca2+ and Na+ in mouse embryonic fibroblast, HeLa, and J774 cells. TPC2-A1-N did not only increase Ca2+ levels in WT but also in all cells with genetically inactivated TPCs. Depletion of Ca2+ from the endoplasmic reticulum (ER) via thapsigargin caused a massive reduction of the TPC2-A1-N induced Ca2+ elevation in all cell lines, indicating that ER plays a key role in this context. Furthermore, our results point to an inositol triphosphate receptor-independent TPC2-A1-N mediated Ca2+ release. Ca2+ depletion from ER was also observed by using an ER-targeted GCaMP6 construct. TPC2-A1-N also raised Na+ levels in mouse embryonic fibroblast cells deficient for TPC1 and TPC2. In summary, our results suggest that TPC2-A1-N induced Ca2+ and Na+ signals are independent of any TPC and that ER represents the major source of Ca2+.
Keywords: Ca(2+) measurement; NAADP; TPC; TPC-A1-N; calcium intracellular release; endo-lysosomal system; endoplasmic reticulum; endosome; intracellular trafficking; ion channel; two-pore channel.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Patel S. Function and dysfunction of two-pore channels. Sci. Signal. 2015;8 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

