Harnessing electroacupuncture: a promising strategy against sleep deprivation-exacerbated post-cardiac arrest brain injury
- PMID: 40784914
- PMCID: PMC12336346
- DOI: 10.1038/s41598-025-15563-y
Harnessing electroacupuncture: a promising strategy against sleep deprivation-exacerbated post-cardiac arrest brain injury
Abstract
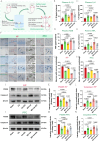
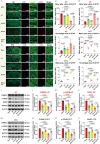
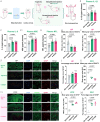
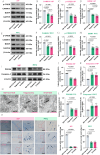
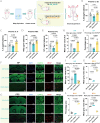
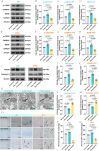
Cardiac arrest (CA)-induced post-cardiac arrest brain injury (PCABI) represents a critical contributor to global mortality and neurological disability. While sleep deprivation (SD) is recognized to aggravate neurological outcomes, its role in PCABI pathogenesis remains underexplored. This study investigated the mechanisms by which SD exacerbates PCABI and evaluated the neuroprotective efficacy of electroacupuncture (EA). A CA model was established in SD rats, followed by RNA sequencing and molecular analyses to assess brain injury biomarkers, synaptic plasticity, and calcium signaling pathways. SD disrupted circadian rhythms, amplified neuronal apoptosis, and suppressed glutamate transporter Excitatory Amino Acid Transporter 2 (EAAT2) expression post-CA, correlating with worsened cognitive deficits. EA treatment significantly attenuated these effects, restoring EAAT2 levels, mitigating calcium overload, and enhancing synaptic integrity. Mechanistically, EA modulated the EAAT2/calcium signaling axis and rebalanced autonomic nervous activity, thereby reducing oxidative stress and neuronal excitotoxicity. These findings identify EAAT2 downregulation as a key mediator of SD-aggravated PCABI and establish EA as a dual-target intervention that rectifies glutamatergic dysregulation and autonomic dysfunction. The study provides translational insights into EA's therapeutic potential for PCABI, particularly in populations with comorbid sleep disturbances.
Keywords: Brain injury; Cardiac arrest; EAAT2; Electroacupuncture; Sleep deprivation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Compliance with ethics requirements: All animal experiment protocols were performed in accordance with the guidelines of Animal Care and Use Committees at the Harbin Medical University (Harbin, Heilongjiang, China) and were in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85 − 23, revised 2011).
Figures

Similar articles
-
Clinical heterogeneity and phenotyping of post cardiac arrest brain injury: one size may not fit all.Intensive Care Med. 2025 Jul;51(7):1240-1255. doi: 10.1007/s00134-025-08012-x. Epub 2025 Jul 16. Intensive Care Med. 2025. PMID: 40668231 Free PMC article. Review.
-
Electroacupuncture Attenuates High-Fat Diet-Exacerbated Alzheimer's Pathology by Enhancing TFEB/TFE3-Mediated Autophagic Clearance of Tau and NLRP3 Inflammasome in 3xTg Mice.CNS Neurosci Ther. 2025 Jul;31(7):e70497. doi: 10.1111/cns.70497. CNS Neurosci Ther. 2025. PMID: 40589337 Free PMC article.
-
Electroacupuncture at ST36 Treatment Suppresses the Microglial Pyroptosis Through Activating α7nAChR in a Rat Model of Asphyxial Cardiac Arrest.J Inflamm Res. 2025 Jul 2;18:8705-8718. doi: 10.2147/JIR.S525373. eCollection 2025. J Inflamm Res. 2025. PMID: 40620602 Free PMC article.
-
Astrocytic YAP prevents the glutamate neurotoxicity by upregulation of EAAT2 expression and promotes the gain of stemness in astrocytes in ischemic stroke mice.Cell Death Dis. 2025 Jul 30;16(1):577. doi: 10.1038/s41419-025-07806-7. Cell Death Dis. 2025. PMID: 40739083 Free PMC article.
-
The use of therapeutic magnesium for neuroprotection during global cerebral ischemia associated with cardiac arrest and cardiac surgery in adults: a systematic review.JBI Database System Rev Implement Rep. 2017 Jan;15(1):86-118. doi: 10.11124/JBISRIR-2016-003236. JBI Database System Rev Implement Rep. 2017. PMID: 28085730
References
-
- Perkins, G. D. et al. Brain injury after cardiac arrest. Lancet398 (10307), 1269–1278 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

